Identify each of the properties illustrated in Problems 2732. x2+x-1 x2+x-1 x2-1 x2-1
Question:
Identify each of the properties illustrated in Problems 27–32.
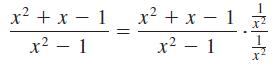
Transcribed Image Text:
x2+x-1 x2+x-1 x2-1 x2-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

Aqib Parvej
I am teaching since my graduation time so I have teaching experience of about 5 years and in these years I learn to teach in the best and interesting way .
4.80+
20+ Reviews
41+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify each of the properties illustrated in Problems 2732. 15 + [a + (-a)] = 15 + 0
-
Identify each of the properties illustrated in Problems 2732. a.mustard + catsup = catsup + mustard commutative b. (red + blue) + yellow = red + (blue + yellow)
-
Identify each of the properties illustrated in Problems 2732. a. a + (10 + b) = (a + 10) + b b. a + (10 + b) = (10 + b) + a
-
Laura Leasing SA signs an agreement on January 1, 2022, to lease equipment to Plote AG. The following information relates to this agreement. 1. The term of the non-cancelable lease is 3 years with no...
-
Caught in an avalanche, a skier is fully submerged in flowing snow of density 96 kg/m3. Assume that the average density of the skier, clothing, and skiing equipment is 1020 kg/m3. What percentage of...
-
Assume that you have been assigned to the audit of Keystone after audit planning has occurred. Review the planning information on pages 235242 and the audit program for the accounts receivable and...
-
Dust mite allergies. A dust mite allergen level that exceeds 2 micrograms per gram 1mg>g2 of dust has been associated with the development of allergies. Consider a random sample of four homes, and...
-
Each of the following compounds is characterized by a 1H NMR spectrum that consists of only a single peak having the chemical shift indicated. Identify each compound. (a) C8H18; 0.9 ppm (f) C2H3Cl3;...
-
last one can i please get some help!! Entries for Notes Receivable, including Year-End Entries The following selected transactions were completed by Fasteners Inc. Co, a supplier of buttons and opers...
-
Find Figure 5.18 shows the cover of a leading mathematics journal. It depicts the symbols for the twelve animals in the Chinese zodiac. (2013, for example, is the year of the snake and 2014 is the...
-
Identify each of the properties illustrated in Problems 2732. a. 5 1 = 1 5 b. 3(4 + 8) = 3 (4) + 3(8)
-
In Problem find each limit. Use - and when appropriate. 2 + 2 -f(x) (x + 2)? (A) lim f(x) (B) lim f(x) (C) lim f(x) X-2 x-2
-
Tabletop Exercise (15%) Develop a tabletop exercise for your organization or community. The size and scope of your exercise can be whatever you need it to be in order for you to complete the...
-
Within the framework of the Porter Five-Forces Model of Competition, describe the competitive force of rivalry among competing sellers. What are some of the factors that increase the rivalry among...
-
The XYZ Corporation has decided to make some changes to help with the work-life balance of its employees. Currently, the organization has 40 employees: 25 full-time employees and 15 part-time...
-
Grocery prices tend to play a role in how people view inflation because of how frequent these purchases are for households. In the past four years grocery prices have jumped 25% which passes overall...
-
Bogg County is a rural area whose residents rely on farming for income. The most popular crop in Bogg County is tobacco, a very labor-intensive plant. To save money, many farmers employ Hispanic...
-
The probability it leaves is 0.03 each second.
-
After looking at the resources, explain what a spirit image is. Why might looking at a god and/or a human in terms of their spirit be helpful if you want to eliminate some of the divisions between...
-
A cigarette lighter contains 2.90 g of butane, C 4 H 10 . Calculate the heat (in kJ) associated with the complete combustion of the butane in the lighter. C 4 H 10 (g) + 13/2 O 2 (g) 4 CO 2 (g) + 5...
-
Calcium metal reacts with hydrochloric acid according to the balanced equation: Ca(s) + 2 HCl (aq) CaCl 2 (aq) + H 2 (g) A 0.150 g sample of Ca metal is combined with enough HCl to make 100.0 mL of...
-
Use the standard enthalpies of formation to determine H rxn for the reaction: Zn(s) + 2 HCl(aq) ZnCl 2 (aq) + H 2 (g)
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


