When 1-cyclobutylethanol (shown below) is treated with concentrated H 2 SO 4 at 120 C, one of
Question:
When 1-cyclobutylethanol (shown below) is treated with concentrated H2SO4 at 120 °C, one of the products that is formed is methylcyclopentene. Please write a mechanism that can account for the formation of this new product.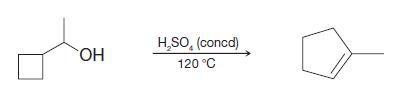
Transcribed Image Text:
H,SO, (concd) HO. 120 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
The most plausible mechanism is given by the attached work sheet can be written in a more general fo...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
When diethyl ether (CH3CH2OCH2CH3) is treated with concentrated HBr, the initial products are CH3CH2Br and CH3CH2OH. Propose a mechanism to account for this reaction.
-
Write mechanisms that account for the products that are formed in the crossed Claisen condensation illustrated earlier between ethyl phenylacetate and diethyl carbonate.
-
When (S)-(+)-1-chloro-2-methylbutane reacts with chlorine, one of the products formed is 1,4-dichloro-2-methylbutane. Does this product have the R or the S configuration?
-
What type of motor would you specify for a meat grinder if the motor is to be exposed?
-
Name three types of capital and explain the differences among them.
-
At the beginning of last year you invested $30,000 in 1,500 shares of Goran Products Inc. During the year you received $4,500 as a dividend. At the end of the year you sold the shares for $18 each....
-
What were the values for the mean and standard deviation for the original sample?
-
A 5.00-F capacitor is initially chmged to a potential of 16.0 V. It is then connected in series with a 3.75-mH inductor. (a) What is the total energy stored in this circuit? (b) What is the maximum...
-
Problem II 10 Points The beginning inventory and purchases of an item for the period were as follows: Beginning inventory 6 units at $73 each First purchase 10 units at $72 each Second purchase 18...
-
An HTTP client opens a TCP connection using an initial sequence number (ISN) of 14,534 and the ephemeral port number of 59,100. The server opens the connection with an ISN of 21,732. Show the three...
-
Working backward, deduce the starting material that led to the indicated product through the defined reactions (A) (B) (1) NANH, (2 equiv) A (2) Li/NH,
-
(a) Synthesize (3S,4R)-3,4-dibromo-1-cyclohexylpentane (and its enantiomer, since a racemic mixture will be formed) from ethyne, 1-chloro-2-cyclohexylethane, bromomethane, and any other reagents...
-
Jumpin Jehosa Phats was incorporated on January 1, 2012 and a year later it needs $10,000,000 to expand operations. JJ Phats is the sole shareholder of the corporation. The corporation is considering...
-
Perpetual Inventory Control Record Description: M & B Supreme Date Purchase Received Issued Sales Units Unit Cost June 1 Balance forward 3 $10.00 4 2 6 8 9 $10.50 9 12 32 3 6 2 4 15 6 10 $11.00 18 20...
-
A rectangular footing of size 4m by 5m is founded at 2m below ground level in a uniform deposit of saturated clay. The footing is designed to support a total vertical load of 8000 kN inclusive of the...
-
P6.2 At the start of Tom Stoppard's "Rosencrantz and Guildenstern are dead" 1, Rosencrantz finds a coin. Guildenstern watches as Rosencrantz repeatedly tosses the coin and every time it comes down...
-
For the data: 9 5 10 7 9 10 11 8 12 769 a) Compute the z-score for the raw score of 10 b) Find the raw score that corresponds to z=+1.22
-
(11%) Problem 7: After a bad thunderstorm, a loose power line comes to rest on a parked van. The van is insulated from the ground by its tires, and accumulates an electric charge of Q = 0.0012...
-
The specification for the weight of a chemical in a compound is 0.06. If the standard deviation of the weighing scales is 0.02, is the process considered capable?
-
Answer the following two independent questions. a. MM Corporation is considering several proposed investments for the coming budget year. MM produces electrical apparatus for industrial complexes....
-
Show a MO energy level diagram for the neutral molecule HeH. Use this diagram to explain whether HeH is expected to stable or not?
-
What is the hybridization at each nitrogen of the amino acid histidine? What kind of orbital is occupied by the unshared pair of electrons on each nitrogen? Explain. :N :Z-H H + NH CH, CHCO,
-
At a pH of 10.8, the amino acid arginine exists primarily as the following dipolar ion. Show the resonance structures for the cationic part of arginine and discuss their relative contributions to the...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


