An unknown compound gives the following mass, IR, and NMR spectra. Propose a structure, and show how
Question:
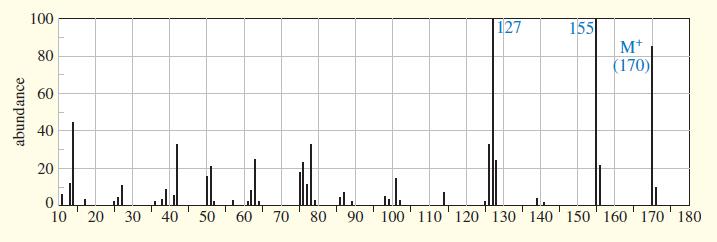
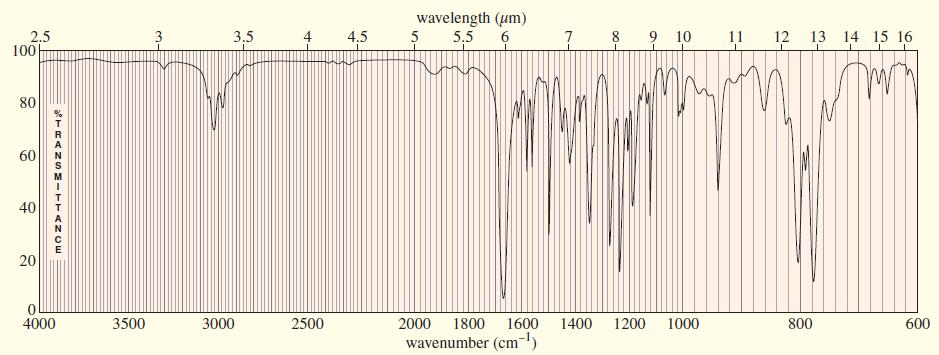
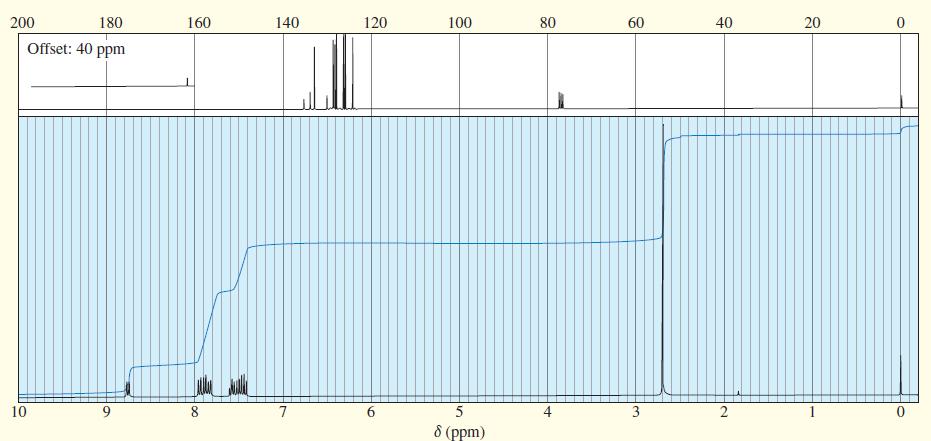
An unknown compound gives the following mass, IR, and NMR spectra. Propose a structure, and show how it is consistent with the spectra. Show the fragmentations that give the prominent peaks at m>z 127 and 155 in the mass spectrum.
Transcribed Image Text:
100 155 M+ (170) 127 80 60 40 20 10 20 40 30 50 60 70 80 ' 90 '100 ' 110 120' 130' 140 150' 160' 170 180 abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
The mass spectrum shows a molecular ion at 170 This serves as the molecular weight for the compound While as is typical the fragmentation is complex t...View the full answer

Answered By

NEHA SINGH
I have done my bacheoler' from DEEN DAYAL UAPADHAYAY GORAKHPUR UNIVERSITY. I am pursuing my master's from IIT BHU. I am former online tutor on CHEGG INDIA. I really enjoying to teach and help students .it is so satisfactory
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The elemental analysis of an unknown compound gives the following results: 74.99 % C, 5.034 % H, 19.98 % O. What is the empirical formula of the unknown compound
-
An unknown compound gives the NMR, IR, and mass spectra shown next. Propose a structure, and show how it is consistent with the observed absorptions. Show fragmentations that account for the...
-
An unknown compound gives a mass spectrum with a weak molecular ion at m/z 113 and a prominent ion at m z 68. Its NMR and IR spectra are shown here. Determine the structure, and show how it is...
-
How are financial statements adjusted for exchange rates?
-
Write the Kb reactions for pyridine and for sodium 2 - mercap - toethanol. ON HOCH,CH,S: Na Pyridine Sodium 2-mercaptoethanol
-
Identify three potential problems with people that may hinder successful implementation of a quantitative model.
-
The method for selecting a stratified sample is to order a population in some way and then select members of the population at regular intervals.
-
We selected a sample of 14 MTF respondents. We present their number of moving (traffic) violations in the last 12 months along with their residential area (residential area is the independent...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Antuan Company set the following standard costs per unit for its product. Direct...
-
Consuelo Chua, Inc., is a disk drive manufacturer in need of an aggregate plan for July through December. The company has gathered the following data: COSTS Holding cost.................$8/disk/month...
-
A student found an old bottle labeled thymol on the stockroom shelf. After noticing a pleasant odor, she obtained the following mass, IR, and NMR spectra. The NMR peak at 4.8 disappears on shaking...
-
Propose a mechanism for each cyanohydrin synthesis just shown.
-
A man having a weight of 175 lb attempts to hold himself using one of the two methods shown. Determine the total force he must exert on bar AB in each case and the normal reaction he exerts on the...
-
Arrow Company processes a food seasoning powder through its Compounding and Packaging departments. In the Compounding Department, direct materials are added at the beginning of the process, and...
-
The 2017 financial statements of LVMH Moet Hennessey Louis Vuitton S.A. are presented in Appendix C at the end of this book. LVMH is a Paris-based holding company and one of the world's largest and...
-
Repeat Problem 10.E1, except design a packed column using 1-in. metal Pall rings. Do the calculations at the top of the column. Approximate HETP for ethanol-water is \(0.366 \mathrm{~m}\). At...
-
We are separating an ethanol-water mixture in a column operating at atmospheric pressure with a total condenser and a partial reboiler. Constant molal overflow (CMO) can be assumed, and reflux is a...
-
Corporate Social Responsibility Problem The Global Reporting Initiative (GRI) is a networkbased organization that has pioneered the development of the world's most widely used sustainability...
-
The cylinder rotates about the fixed z-axis in the direction indicated. If the speed of point A is v A = 2 ft/sec and the magnitude of its acceleration is a A = 12 ft/sec 2 , determine the angular...
-
Is that Yelp review real or fake? The article A Framework for Fake Review Detection in Online Consumer Electronics Retailers (Information Processing and Management 2019: 12341244) tested five...
-
A small amend of a by4roduct, gdibromobenzene, is also formed in the brorlination of benzene shown in Eq. 16.2. Write a curved-arrow mechanism for formation of this compound. Eq. 16.2 HBr FeBra or Fe...
-
A compound called p-toluenesulfonic acid is formed when toluene is sulfonated at the para position. Draw the structure of this compound, and give the curved-arrow mechanism for its formation.
-
What electrophilic substitution product is formed when 2-methylpropene is added to a large excess of benzene containing HF and the Lewis-acid BF3? By what mechanism is it formed?
-
On an average day, a company writes checks totaling $1,500. These checks take 7 days to clear. The company receives checks totaling $1,800. These checks take 4 days to clear. The cost of debt is 9%....
-
Olds Company declares Chapter 7 bankruptcy. The following are the book values of the asset and liability accounts at that time. A bankruptcy expert estimates that administrative expense will total $...
-
As the representative of the local accounting club, you have been asked by the dean to help her understand the costs of the different degrees offered at the school. You decide to use an...

Study smarter with the SolutionInn App


