Birch reduction of toluene leads to a product with the molecular formula C 7 H 10 .
Question:
Birch reduction of toluene leads to a product with the molecular formula C7H10. On ozonolysis followed by reduction with dimethyl sulfide, the product is transformed into
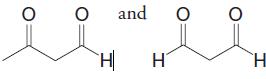
What is the structure of the Brich reduction product?
Transcribed Image Text:
and H H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
ans 1 Methy...View the full answer

Answered By

KATTUBOINA KOTA SATYA SAIKUMAR
EDUCATION;
I qualified CSIR-NET EXAM in december 2019.i got 49th rank in LS category,and also i cleared APSET exam which is conduct by ANDHRA UNIVERSITY in 2018.
M.SC (chemistry) from ANDHRA UNIVERSITY 2016-2018.
B.SC (MPC) from ADIKAVINANNAYYA UNIVERSITY 2013-2016.
Now i am working as chemistry lecturer in s.m.v.m.polytechnic college .
i can teach chemistry in iit-jee level and also i solve chemistry problems as simple as possible and give tricks to students for solve problems.i also help to students for enhancing their chemistry subject.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118133576
11th edition
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
Question Posted:
Students also viewed these Sciences questions
-
There are three constitutional isomers with the molecular formula C 5 H 12 . Chlorination of one of these isomers yields only one product. Identify the isomer, and draw the product of chlorination.
-
Sabinene and 3-carene are isomeric natural products with the molecular formula C10H16. (a) Ozonolysis of sabinene followed by hydrolysis in the presence of zinc gives compound A. What is the...
-
Sabinene and 3-carene are isomeric natural products with the molecular formula C10H16. (a) Ozonolysis of sabinene followed by hydrolysis in the presence of zinc gives compound A. What is the...
-
As discussed throughout Chapter 3, the owner of Evergreen Solar (Jennifer) has been exploring different ways of performing predictive analytics in order to better predict whether any new sales lead...
-
The diprotic acid H2A has pK1 = 4.00 and pK2 = 8.00. (a) At what pH is [H2A] = [HA-]? (b) At what pH is [HA-] = [A2-]? (c) Which is the principal species at pH 2.00: H2A, HA-, or A2-? (d) Which is...
-
Trace the historical roots of critical approaches. Do you think critical theorizing is still relevant today? Why or why not?
-
Study of sex offenders. A study of sex offenders in the Canadian Federal Prison System was published in the British Journal of Criminology (May 2014). The following data were collected for each of 59...
-
At December 31, 2014, Townlynn Corporation reported the stockholders equity accounts shown here (with dollar amounts in millions, except per-share amounts). Common stock $2.00 par value per share,...
-
During 2020, Pepe Guardio purchases the following property for use in his calendar year-end manufacturing business: Item Date Acquired Cost Manufacturing equipment (7 year) June 2 $50,000 Office...
-
Wayland Custom Woodworking is a firm that manufactures custom cabinets and woodwork for business and residential customers. Students will have the opportunity to establish payroll records and to...
-
Compound E has the spectral features given below. What is its structure? MS (m/z): M 202 IR (cm -1 ): 30303080, 2150 (very weak), 1600, 1490, 760, and 690 1 H NMR (): narrow multiplet centered at...
-
The reactions of aldehydes and ketones with LiAlH 4 and NaBH 4 (Section 12.3) are nucleophilic additions to the carbonyl group. What is the nucleophile in these reactions?
-
1. What are the specific requirements for a court to grant specific performance to SWB? 2. Do these requirements fit this case?
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
3.6. Explain and discuss the potential benefits to be gained by using blade twist, plan- form taper, low solidity, large radius, and low rotational speed for the main rotor of a heavy lift helicopter...
-
2. A VRM (Voltage Regulator Modul) is used to supply the voltageto the CPU of a computer. In the new generation of microprocessors,whose power consumption is 100W, the input voltage to the VRM is12V...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
The shaft of the wheel unit rolls without slipping on the fixed horizontal surface, and point O has a velocity of 3 ft /sec to the right. By the method of this article, determine the velocities of...
-
In Exercises find dy/dx by implicit differentiation. xy - y = x
-
(a) Draw the resonance forms for SO2 (bonded O-S-O) (b) Draw the resonance forms for ozone (bonded O-O-O) (c) Sulfur dioxide has one more resonance form than ozone. Explain why this structure is not...
-
There is a small portion of the periodic table that you must know to do organic chemistry. Construct this part from memory, using the following steps. (a) From memory, make a list of the elements in...
-
For each compound, state whether its bonding is covalent, ionic, or a mixture of covalent and ionic. (a) NaCl (b) NaOH (c) CH3Li (d) CH2CI2 (e) NaOCH3 (f) HCO2Na (g) CF4
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


