Cholesterol has the following structure. Tell whether the - OH group is axial or equatorial.
Question:
Cholesterol has the following structure. Tell whether the -OH group is axial or equatorial.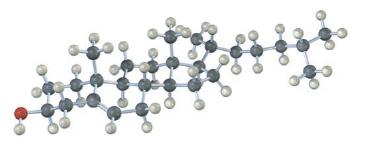
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
In the given structure of cholesterol the OH group hydroxyl group is equatorial In cycloh...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw each molecule in a stable chair conformation, and tell whether each red group is axial or equatorial. (a) (b) (c) (d) CH CH3 CH CH3 l1 C H,C H HIH OH H androsterone H.C H,C H HOH HO...
-
Consider the two stereo isomers of 1, 4-dimethyl cyclohexane: (a) Explain whether each methyl is axial or equatorial in the conformations of the cis-isomer. (b) Explain whether each methyl is axial...
-
Tell whether each structure or term is a correct description of the L-sorbose structure shown here or a form with which it is in equilibrium. (b) a ketohexose (d) A ketohexose (f) An aldohexose CH2OH...
-
The exact perimeter P of a square is 50 feet. What measured lengths are possible for the side S of the square to have relative error in the perimeter that is less than or equal to 0.04 (or 4%)?
-
Consider the following costs that were incurred during the current year: 1. Advertising costs of Nike. 2. Straight- line depreciation on factory machinery of Airbus Industrie. 3. Wages of assembly-...
-
Use a triple integral to find the volume of the solid bounded by the graphs of the equations. z = xy, z = 0, 0 x 3, 0 y 4
-
The mean of the y-values
-
Gant Company reported net income of $157,000. It reported depreciation expense of $12,000 and accumulated depreciation of $47,000. Amortization expense was $8,000. Gant purchased new equipment during...
-
Fashion accessories Ltd produces a high-quality fashion accessories that passes through two production processes. Data for June for the first process follow: Work in process inventory, June 1 50,000...
-
Eleostearic acid, C 18 H 30 O 2 , is a rare fatty acid found in tung oil. On oxidation with KMnO 4 , eleostearic acid yields 1 part pentanoic acid, 2 parts oxalic acid (HO 2 COCO 2 H), and 1 part...
-
Write the structures of the following molecules: (a) Sodium stearate (b) Ethyl linoleate (c) Glyceryl dioleopalmitate
-
Explain how to construct (a) A cause-and-effect diagram. (b) A defect concentration diagram.
-
Jason is a sole trader in the architecture industry. For the year ending 30 June 2019, Jason hired a 3D model designer, Sarah, to help him with the growing business. At the end of the year he has the...
-
Read Case 14-1 Trojan Technologies (15th ed., p. 426 OR 16th ed., p. 431) Guiding Questions and additional information: In preparing your case study, ensure that you answer the following questions:...
-
Jorge Rimert works for Road to Success Collection Agency. He oversees mailing out collection notices to patients. Upon review of the patients who have not paid from Hideaway Hospital, Jorge notices...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 99% confident that the estimated percentage is in error...
-
A 100 acre plot of land has a concentration time of 80 minutes. The area is residential single family homes with a C-0.40. What is the percent Increase in stormwater runoff from a 50 year recurrence...
-
A firm builds wooden shipping crates. How does the cost of producing a 1-cubic-foot crate (each side is 1 foot square) compare to the cost of building an 8-cubic-foot crate if wood costs $1 per...
-
Don Griffin worked as an accountant at a local accounting firm for five years after graduating from university. Recently, he opened his own accounting practice, which he operates as a corporation....
-
Methyl p-nitrobenzoate has been found to undergo saponification faster than methyl benzoate. (a) Consider the mechanism of saponification, and explain the reasons for this rate enhancement. (b) Would...
-
A student has just added ammonia to hexanoic acid and has begun to heat the mixture when he is called away to the telephone. After a long telephone conversation, he returns to find that the mixture...
-
In Section 21-16, we saw that Sevin insecticide is made by the reaction of 1-naphthol with methyl isocyanate. A Union Carbide plant in Bhopal, India, once used this process to make Sevin for use as...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


