Draw a Lewis structure, and classify each of the following compounds: (a) CH 3 CH 2 CONHCH
Question:
Draw a Lewis structure, and classify each of the following compounds:
(a) CH3CH2CONHCH3
(b) (CH3CH2)2NH
(c) (CH3)2CHCOOCH3
(d) CH3CHCHCOCl
(e) (CH3CH2)2O
(f) CH3CH2CH2CN
(g) (CH3)3CCH2CH2COOH
(h)
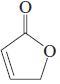
(i)
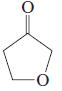
(j)
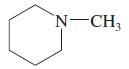
(k)
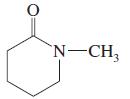
(l)
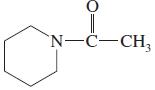
(m)
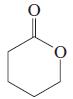
(n)
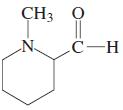
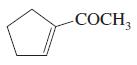
(p)
Transcribed Image Text:
`N-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
1 2 3 4 5 6 CH CH CO NH CH HH HCCCNCH HH HH0 CH CHNH H CH5NCH5 CH CH COOCH HC HC ...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a Lewis structure, and classify each of the following compounds. The possible classifications are as follows: (a) CH 2 CHCHO (b) CH 3 CH 2 CH(OH)CH 3 (c) CH 3 COCH 2 CH 3 (d) CH 3 CH 2 OCHCH 2...
-
Draw a Lewis structure for each of the following compounds: (a) C 2 H 6 (b) C 2 H 4 (c) C 2 H 2 (d) C 3 H 8 (e) C 3 H 6 (f) CH 3 OH
-
Draw a Lewis structure for each of the following species. Again, assign charges where appropriate. (a) H- (b) CH3- (c) CH3+ (d) CH3 (e) CH3NH3+ (f) CH3O- (g) CH2 (h) HC2-(HCC) (i) H2O2 (HOOH)
-
For each equation: a) Tell whether the equation describes a parabola, an ellipse, or a hyperbola. b) State whether the directrix is vertical or horizontal and give its location in relation to the...
-
Calculate how many milliliters of 0.626 M KOH should be added to 5.00 g of MOBS (Table 8-2) to give a pH of 7.40.
-
Consider the steps in the research process. What are some of the major problems that are likely to be encountered at each step in the research process?
-
Violent behavior in children. Refer to the Development Psychology (Mar. 2003) study of the behavior of elementary school children, presented in Exercise 8.149 (p. 454). The researchers used a...
-
Selected transactions completed by Hirata Company during its first fiscal year ending December 31 were as follows: Jan. 2. Issued a check to establish a petty cash fund of $1,400. Mar. 1. Replenished...
-
Suppose that $140,000 is owed on a house after the down payment is made. The monthly payment for principal and interest at 9.5% for 30 years is 140 8.40854 = $1177.20 What is the total amount that...
-
On February 1, Williams Storage agreed to rent Haka Manufacturing warehouse space for $4,000 per month. Haka Manufacturing paid the first three months rent in advance. a. Prepare the necessary...
-
Classify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications: (a) (CH 3 CH 2 ) 2 CHCH(CH 3 ) 2 (b) CH 3 CHCHCH...
-
If the carbon atom in CH 2 Cl 2 were flat, there would be two stereoisomers. The carbon atom in CH 2 Cl 2 is actually tetrahedral. Make a model of this compound, and determine whether there are any...
-
What are the advantages of nonstatistical sampling over statistical sampling?
-
Write a java program that contain two overloaded methods that accepts two numbers or two characters representing a range example (11, 37) or (c, w) inputted by the user. The method generates a random...
-
Maggie could not conceive a child using natural means, so she sought out a woman who would donate an egg to be surgically implanted in Maggie. Which of the following items are deductible by Maggie in...
-
M corporation is subject to tax only in state b state b law provides for the use of federal taxable income before net operating loss and special deductions as the starting point for computing state...
-
Use Routh Criteria to determine the values of K needed for the system represented by the Characteristic Equation to be stable. (1 + K)s + (2K + 3)s + 2 3K = 0 Obtain the root locus plot for the...
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
At the instant represented, the velocity of point A of the 1.2-m bar is 3 m/s to the right. Determine the speed v B of point B and the angular velocity w of the bar. The diameter of the small end...
-
The rate at which the temperature of an object changes is proportional to the difference between its own temperature and the temperature of the surrounding medium. Express this rate as a function of...
-
Which of the ring-opening reactions given in Fig. Pl 1.48 should occur most readily? Explain.
-
Explain how you could differentiate between the compounds in each of the following pairs by using simple physical or chemical tests that give readily observable results, such as obvious solubility...
-
Explain how you could differentiate between the compounds in each of the following pairs by using simple physical or chemical tests that give readily observable results, such as obvious solubility...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


