Explain why the ether obtained by treating an optically active alcohol with PBr 3 followed by sodium
Question:
Explain why the ether obtained by treating an optically active alcohol with PBr3 followed by sodium methoxide has the same configuration as the alcohol, whereas the ether obtained by treating the alcohol with tosyl chloride followed by sodium methoxide has a configuration opposite that of the alcohol.
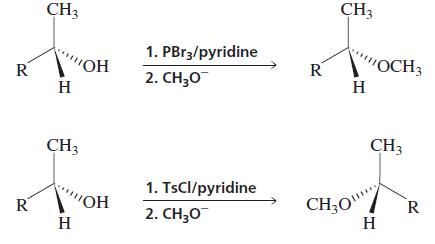
Transcribed Image Text:
CH3 CH3 1. PBr3/pyridine R OH R "OCH3 H 2. СH,0 H CH3 CH3 1. TsCl/pyridine CH;O" H R VOH R 2. CH;0 H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
When the ether obtained by treating the optically active alcohol with PBr 3 followed by s...View the full answer

Answered By

Shakil Ahmed
I hold a Master's degree in Chemistry. I have a special interest in organic and inorganic chemistry. I have worked as a teaching assistant for undergraduate students for a semester. I focus on strengthening the basic concept first as it will help throughout.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain the following observations: When tert-butyl bromide is treated with sodium methoxide in a mixture of methanol and water, the rate of formation of tert-butyl alcohol and tert-butyl methyl...
-
Explain why the following are not optically active: a. The product obtained from the reaction of 1,3-butadiene with cis-1,2-dichloroethene b. The product obtained from the reaction of 1,3-butadiene...
-
When the following optically active alcohol is treated with HBr, a racemic mixture of alkyl bromides is obtained: Draw the mechanism of the reaction, and explain the stereochemical outcome. Br HBr +...
-
In the figure below, which compares different DNA extraction kits (e.g, MO BIO and PSP), which one of the two trees shows the co-occurrence of microbial taxa? Proportion of max 0 0.2 0.4 0.6 0.8...
-
Find the minimum angle i for total reflection in the optical fiber in Figure 19-20 if the index of refraction of the cladding is 1.400 and the index of refraction of the core is (a) 1.600 or (b)...
-
What are Kohlbergs three levels of moral behavior development? LO.1
-
View of rotated objects. Perception & Psychophysics (July 1998) reported on a study of how people view threedimensional objects projected onto a rotating two- dimensional image. Each in a sample of...
-
Llano Lamps has the following revenue and cost functions: Revenue = $ 70 per unit Cost = $ 90,000 + $ 40 per unit a. What is the break-even point in units? b. What is the break-even point in dollars?
-
Valley Spa purchased $10,700 in plumbing components from Tubman Co. Valley Spa signed a 120-day, 9% promissory note for $10,700. If the note is dishonored at maturity, what is the amount due on the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Why isnt a cumulated diene formed in the preceding reaction?
-
Show, using any necessary reagents, how the following compounds could be prepared with ethylene oxide as one of the reactants: a. CH 3 CH 2 CH 2 CH 2 OH b. CH 3 CH 2 CH 2 CH 2 D c. CH 3 CH 2 CH 2 CH...
-
Determine the competitive relevance of each strength and weakness with the aid of a series of carefully formulated questions.
-
Country Analysis of Milan: -Discuss the overall cultural, political, legal, economic, and technological infrastructure issues that could potentially impact a product's introduction in Milan. -You...
-
Sarah is a highly introverted employee who works in a fast-paced sales team. Her manager recently noticed that Sarah tends to be reserved during team meetings and rarely volunteers her ideas....
-
Community Disaster Preparedness Review the information found at https://www.ready.gov/community-preparedness-toolkit Look at the steps involved in formulating community response to a disaster....
-
The topic of diversity, tolerance, and inclusion has been widely (and, in many cases, hotly) debated in society over the past several years. In my opinion (and this is up for discussion - hence this...
-
The objective of this coursework is for you to critically engage with the theory and practice of promoting wellbeing or equality, diversity and inclusion (including cultural diversity) for employees...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. C.C er+y* sin y dx dy = 0 -1 Jo
-
Two mutually exclusive investment alternatives are being considered. Alternative A requires an initial investment of $20,000 in a machine. Annual operating and maintenance costs are anticipated to be...
-
Without drawing the MOs, state whether the 7r-molecular orbital 6 in 1,3,5,7,9-decapentaene (a 10-carbon conjugated alkene) is symmetric or anti symmetric with respect to the reference plane; is...
-
What do the pericyclic selection rules have to say about the position of equilibrium in each of the reactions given in Fig. P27.30? Which side of each equilibrium is favored and why? Fig. P27.30 (a)...
-
What stereoisomer of A also gives compound C on heating?
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


