Question: For each compound, 1. Name the functional group. 2. Show what compound(s) result from complete hydrolysis. a. b. c. d. e. f. g. h. CH,0
For each compound,
1. Name the functional group.
2. Show what compound(s) result from complete hydrolysis.
a.
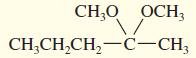
b.
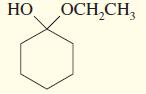
c.
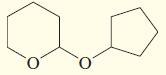
d.
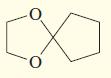
e.
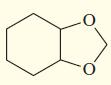
f.
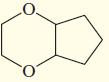
g.
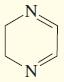
h.
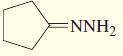
CH,0 OCH; CH;CH,CH,-C-CH;
Step by Step Solution
3.44 Rating (176 Votes )
There are 3 Steps involved in it
a ether functional group 0 b hydroxyl OH ... View full answer

Get step-by-step solutions from verified subject matter experts


