-Glucose has the following structure. Identify the chirality centers in -glucose, and tell how many stereoisomers of...
Question:
β-Glucose has the following structure. Identify the chirality centers in β-glucose, and tell how many stereoisomers of glucose are possible.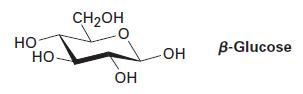
Transcribed Image Text:
НО НО CH₂OH ОН -ОН OH B-Glucose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
In glucose the structure is HO CH2OHCOH OH glucose has four ch...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify all chirality centers in each of the following compounds: a. b. c. d. e. f. . Ascorbic acid (Vitamin C) Vitamin D3
-
The Fischer projection formula for glucose (blood sugar, Sec. 16.4) is Altogether, how many stereoisomers of this sugar are possible? CH= O HOH HOH CH OH glucose
-
Extract of Ephedra sinica, a Chinese herbal treatment for asthma, contains the compound ephedrine, which dilates the air passages of the lungs. The naturally occurring stereoisomer is levorotatory...
-
Two wheels A and B have masses m A and m B and radii of gyration about their central vertical axes of k A and k B respectively. If they are freely rotating in the same direction at A and about the...
-
A firm produces output according to a production function Q = F (K, L) = min {4K, 8L}. a. How much output is produced when K = 2 and L = 3? b. If the wage rate is $ 60 per hour and the rental rate on...
-
Relate to the function whose graph is sketched in Fig. 12. Find f (0) and f (7). Y (2, 3) A 1 3 5 Figure 12 (7, -1) 9 y = f(x) X
-
Who burns more energy? Table 14.1 gives data on the lean body mass and metabolic rate for 12 women and 7 men. You made a scatterplot of these data in Exercise 14.14. (a) Do you think the correlation...
-
Jose is a general manager of a division of Global Operations. In that capacity, he knows that his company is planning on making layoffs soon. Juan, a good friend in another division, tells Jose he is...
-
Wage and Tax Statement Data on Employer FICA Tax Ehrlich Co. began business on January 2, 20Y8. Salaries were paid to employees on the last day of each month, and social security tax, Medicare tax,...
-
How would you prepare the following alkyl halides from the appropriate alcohols? (a) CI CH3CCH3 CH3 CH3 CH3CHCHCHCH3 (b) Br (c) CH3 T BrCHCHCHCHCHCH3 (d) CH3 CI I I CH3CHCHCHCCH3 CH3
-
Write representative structures for the following: (a) A fat (b) A vegetable oil (c) A steroid
-
Determine the mass and the location of the center of mass (x, y) of the uniform parabolic-shaped rod. The mass per unit length of the rod is 2kg>m. y? = 4x- 4 m -4 m-
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
A procurement officer finds that the delivery time for a specific item is never less than 5 days. The worst case scenario is that it takes 30 days for the item to arrive. A delivery lead time of 10...
-
Show that gj concave AHUCQ Abadie For nonnegative variables, we have the following corollary.
-
The following two compounds are both secondary alkyl halides, but they undergo E2 reactions at different rates. The first compound reacts more rapidly than the second compound. Explain. Br Br
-
The following three reactions are similar, differing only in the configuration of the substrate. One of these reactions is very fast, one is very slow, and the other does not occur at all. Identify...
-
1-Bromobicyclo [2.2.2] octane does not undergo an E2 reaction when treated with a strong base. Explain why not.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


