The following three reactions are similar, differing only in the configuration of the substrate. One of these
Question:
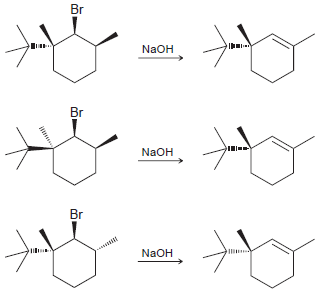
Transcribed Image Text:
Br NaOH Br NaOH Br NaOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (20 reviews)
The first reaction is very slow because the tertbutyl group effectively locks the ring i...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following three reactions: (a) For each of the reactions, use data in Appendix C to calculate H, G, and S at 25C. (b) Which of these reactions are spontaneous under standard conditions...
-
In each of the following three nuclear fusion reactions, the masses of the nuclei are given beneath each nucleus. Rank the energy produced by each reaction in descending order (greatest first). (a)...
-
Three reactions of 2-chloro-2-methylpropane are shown here, (a) Write the major product of each transformation (b) Compare the rates of the three reactions. Assume identical solution polarities and...
-
Blaine is a practicing dentist. He operates his business from the basement of his house, with a separate entrance and facilities. Blaine uses the simplified method every year to calculate his...
-
1. In the light of this chapter, what do you think the ideal form of government would be? Would it be different for a Christian than it would be for someone who is not a Christian? 2. What do you...
-
Factor the given expressions completely. 64 x 6
-
About what percentage of U.S. companies include customer satisfaction as a measure of salesperson performance? (check one) 10% 30% 50% 20% 40% 60%
-
Tess is the development manager for the Isabelle Stewart Gardner Museum in Boston. She was in the middle of a large campaign to raise $50 million for a building expansion project. Her development...
-
Insurance expense $10,000 Sales returns and allowances 22,400 Bad debt expense 6,000 Accounts payable 81,000 Accounts receivable 108,590 Allowance for doubtful accounts 8,500 Accumulated depreciation...
-
Refer to Figure 10.45. A flexible circular area of radius 6 m is uniformly loaded by q = 450 kN/m2. Using Newmark's chart, determine the increase in vertical stress, z, at point A. Plan 4 450 kN/m2 6...
-
The following two compounds are both secondary alkyl halides, but they undergo E2 reactions at different rates. The first compound reacts more rapidly than the second compound. Explain. Br Br
-
1-Bromobicyclo [2.2.2] octane does not undergo an E2 reaction when treated with a strong base. Explain why not.
-
Refer to the partially completed payroll register you started at the end of Chapter 2. You will now determine the amount of FICA taxes to be withheld from each employees pay for the pay period ending...
-
Encouraging you to sit back and watch a full hour of one of your favorite shows on prime-time television. However, instead of getting up during the commercial break or fast forwarding through the...
-
A family member has been recently diagnosed with a heart condition that requires replacing a heart valve. She points out that if she goes to India, the surgery cost is about 60% cheaper on average...
-
Based on the case of Bowers Machine Parts. Critically analyze why people were not doing their best and critically explain why hiring a consultant might solve the issue. Justify your answer by using...
-
Identify a few strategies for sustainability effectiveness. Should sustainability be a corporation's top priority? Why or why not? What are the challenges associated with implementing sustainable...
-
Answer the following questions for the topic you want to write about. Type your answers in a separate Word document. What is the issue or debatable idea you might write about? What is debatable about...
-
In Exercises 9 through 22, find the intervals of increase and decrease for the given function. G(x) = x - 12/1/2
-
Can partitioned join be used for r r.A s? Explain your answer
-
Propose structures for compounds that fit the following data: (a) A ketone with M = 86 and fragments at m/z = 71 and m/z = 43 (b) An alcohol with M + = 88 and fragments at m/z = 73, m/z = 70, and m/z...
-
2-Methylpentanc (C 6 H 14 ) has the mass spectrum shown. Which peak represents M + ? Which is the base peak? Propose structures for fragment ions of m/z = 71, 57, 43, and 29. Why does the base peak...
-
Assume that you are in a laboratory carrying out the catalytic hydrogenation of cyclohexane to cyclohexane. How could you use a mass spectrometer to determine when the reaction is finished?
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


