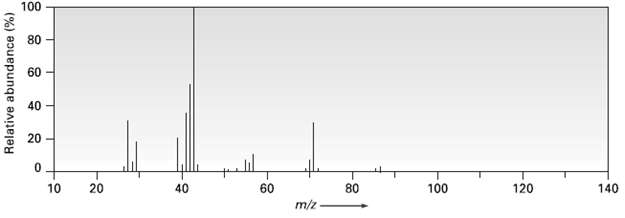
2-Methylpentanc (C 6 H 14 ) has the mass spectrum shown. Which peak represents M + ?...
Question:
2-Methylpentanc (C6H14) has the mass spectrum shown. Which peak represents M+? Which is the base peak? Propose structures for fragment ions of m/z = 71, 57, 43, and 29. Why does the base peak have the mass it does?
Transcribed Image Text:
100 80 20 120 40 10 140 20 60 80 100 m/z Relative abundance (%) 20 8 유
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
CHCHCHCHCH mz 71 CH3 CH3CHCHCHCH3 2Methylpentane k ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following mass spectrum is for octane. a) Which peak represents the molecular ion? b) Which peak is the base peak? c) Draw the structure of the fragment that produces the base peak. 100 80 60 40...
-
Propose structures and fragmentation mechanisms corresponding to ions with m/z 57 and 41 in the mass spectrum of 4-methyl-1-hexene. onization (loss of a electron) mlz 57 m/z 41
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
Solar Energy Corp. has $4 million in earnings with four million shares outstanding. Investment bankers think the stock can justify a P/E ratio of 21. Assume the underwriting spread is 5 percent. What...
-
In the United States, the incomes of specialists such as heart surgeons can easily triple the incomes of primary care practitioners. Use the Five Forces to offer explanation for this disparity. Can...
-
A loudspeaker at the origin emits a 120 Hz tone on a day when the speed of sound is 340 m/s. The phase difference between two points on the x-axis is 5.5 rad. What is the distance between these two...
-
Suppose the Robert H. Smith School of Business at the University of Maryland would like to compare the starting salaries for both men and women who graduated with different majors. The following data...
-
The physically realizable form of the PD transfer function is given in the first equation of Exercise 8.1.(a) Show how to obtain this transfer function with a parallel arrangement of two much simpler...
-
Decker Tires free cash flow was just FCF0 = $1.32 million. Analysts expect the company's free cash flow to grow by 30% this year, by 10% in Year 2, and at a constant rate of 6% in Year 3 and...
-
What will your portfolio be worth in 10 years? In 20 years? When you stop working? The Human Resources Department at Tri-State Corporation was asked to develop a financial planning model that would...
-
Propose structures for compounds that fit the following data: (a) A ketone with M = 86 and fragments at m/z = 71 and m/z = 43 (b) An alcohol with M + = 88 and fragments at m/z = 73, m/z = 70, and m/z...
-
Assume that you are in a laboratory carrying out the catalytic hydrogenation of cyclohexane to cyclohexane. How could you use a mass spectrometer to determine when the reaction is finished?
-
A corporation desires to enter a particular foreign market. The direct foreign investment analysis indicates that a direct investment in the plant in the foreign country is not profitable. What other...
-
Consider the following thermochemical equation: 2 Na 2 O 2 (s) + 2 H 2 O(l) 4 NaOH(s) + O 2 (g) H = -126 kJ Calculate the enthalpy change when 41.5 g of Na 2 O 2 with water?
-
Newton's Laws Introduction Problems
-
Mr. A and B agreed to start a business agreed to share profit and loss based the condition that will profit only when there is profit in excess of BD 10,000 this from of business is called as:...
-
L= {a'e"b"d' | i=1+m and l,m,n 20] a. Write at least 10 strings of the above language in increasing order of string length. b. Write Context Free Grammar (CFG) for the above language.
-
The Beta Co. shows the following results of operation on Dec. 31. Variable cost Fixed costs Direct materials P512,500 Direct labor 575,000 Manufacturing overhead 400,000 P212,500 For the year then...
-
Understand the role of conceptualization in research
-
The Strahler Stream Order System ranks streams based on the number of tributaries that have merged. It is a top-down system where rivers of the first order are the headwaters (aka outermost...
-
The osmotic pressure of a solution containing 2.10 g of an unknown compound dissolved in 175.0 mL of solution at 25 C is 1.93 atm. The combustion of 24.02 g of the unknown compound produced 28.16 g...
-
Show how you might synthesize the following compounds, using acetylene and any suitable alkyl halides as your starting materials. If the compound given cannot be synthesized by this method, explain...
-
Show how you would synthesize each compound, beginning with acetylene and any necessary additional reagents. (a) prop-2-yn-1-ol (propargyl alcohol) H----C === C--- CH2OH (b) (c) (d) hept-2-yn-4-ol OH...
-
Show how you would synthesize 2-phenylhex-3-yn-2-ol, starting with acetophenone (PhCOCH3) and any other reagents you need. ("2-ol" means there is an OH group on C2.)
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...
-
Regarding Enron, this was a company that resulted in the creation of the Sarbanes-Oxley Act and many reforms to the accounting profession. Research the company and answer the following...

Study smarter with the SolutionInn App


