The following mass spectrum is for octane. a) Which peak represents the molecular ion? b) Which peak
Question:
a) Which peak represents the molecular ion?
b) Which peak is the base peak?
c) Draw the structure of the fragment that produces the base peak.
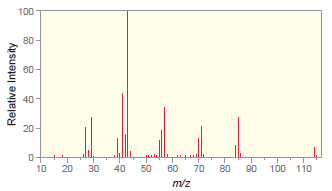
Transcribed Image Text:
100 80 60 40 20 110 10 20 30 40 50 60 70 80 90 100 m/z Relative Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
a The molecular io...View the full answer

Answered By

Nandana Wijayarathna
I am a highly experienced writer in several areas,
Business management
Information technology
Business administration
Literature
Biology
Environmental science
History
4.50+
161+ Reviews
399+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
2-Methylpentanc (C 6 H 14 ) has the mass spectrum shown. Which peak represents M + ? Which is the base peak? Propose structures for fragment ions of m/z = 71, 57, 43, and 29. Why does the base peak...
-
Calculate the mass of octane, C8H18(l), that must be burned in air to evolve the same quantity of energy as produced by the fusion of 1.0 g of hydrogen in the following fusion reaction: Assume that...
-
Use Figure 24-15 to suggest which type of liquid chromatography you could use to separate compounds in each of the following categories: (a) Molecular mass < 2 000, soluble in octane (b) Molecular...
-
In Exercises 7980, find the value of y if the line through the two given points is to have the indicated slope. (3, y) and (1, 4), m = -3
-
Write an essay about a "Magazine" focusing on the demographics, describing the niches the magazine is trying to reach. For this the chosen magazine is: Elle magazine. Some demographics that should be...
-
Solve for the angle A for the given triangles in the given figures in terms of the given sides and angles, explain your method. A b Fig. 20.43 a B
-
2. Using the information in the previous problem, find the price of a 5-year coupon bond that has a par payment of $1,000.00 and annual coupon payments of $60.00.
-
If a capital project is incomplete at the end of a fiscal year, why is it considered desirable to close Encumbrances and all operating statement accounts at year-end? Why is it desirable to...
-
Calculating the Average Total Assets and the Return on Assets The income statement, statement of retained earnings, and balance sheet for Somerville Company are as follows. Also, assume a tax rate of...
-
Mr B aged 52 years, has earned rupees 75,00,000 out of his business. His ex-wife gifted him a car worth rupees 8 lakh. He spent a total of rupees 20 lakh during a family trip. He won a lottery of 16...
-
Propose a molecular formula that fits the following data. a) A hydrocarbon (C x H y ) with a molecular ion peak at m/z = 66 b) A compound that absorbs IR radiation at 1720 cm -1 and exhibits a...
-
Calculate the HDI for each molecular formula. a) C 4 H 6 b) C 5 H 8 c) C 40 H 78 d) C 72 H 74 e) C 6 H 6 O 2 f) C 7 H 9 NO 2 g) C 8 H 10 N 2 O h) C 5 H 7 Cl 3 i) C 6 H 5 Br j) C 6 H 12 O 6
-
Determine the configuration of each of the following alkenes as Z or E as appropriate:
-
what methods ,to do ,or steps do you need to be sure to address when need to make a change in your organization that will help you navigate the organizational culture related to change? Especially...
-
How do you best receive information? Do you prefer written or oral reports? Shorter or longer briefings or reports? Quantitative or qualitative data? Formal or informal styles? How do you ensure...
-
Continuing with an examination of the laws in the state you've written about in earlier discussions, what state and local statutes exist that address the medical conditions or needs of eligible...
-
1. Think about the various soft drinks that you know from the local market and chose any 3 out of that ( e.g. Coca-Cola, Pepsi, 7-Up, Mirinda Citrus, Saudi Champagne, Shaani, Sun Top & Sun Cola,...
-
Leadership is an integral element in any job, regardless of the work title. However, it is important to recognize that leadership is not just one single skill; instead, success in leadership depends...
-
Solve the equation. Check your answers. 3x + 7 = 3x + 5
-
The outer loop controls the number of students. Note that the inner loop of this program is always executed exactly three times, once for each day of the long weekend. Modify the code so that the...
-
Is there an unbranched alkane containing 23 hydrogen atoms? If so, give its structural formula; if not, explain why not.
-
In the structure of 4- isopropy 1-2,4,5-trimethylheptane (Problem 2.9) (a) Identify the primary, secondary, tertiary, and quaternary carbons. (b) Identify the primary, secondary, and tertiary...
-
In the structure of 4- isopropy 1-2,4,5-trimethylheptane (Problem 2.9) (a) Identify the primary, secondary, tertiary, and quaternary carbons. (b) Identify the primary, secondary, and tertiary...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


