How can the following compounds be prepared, starting with a carbonyl compound with one fewer carbon atoms
Question:
How can the following compounds be prepared, starting with a carbonyl compound with one fewer carbon atoms than the desired product?
a. HOCH2CH2NH2
b.
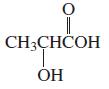
Transcribed Image Text:
CH3CHČOH ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
A this can be prepared by the reaction of methyl c...View the full answer

Answered By

Raju Mandal
From my childhood , i was good in studying. I have completed my 10th exam from sagarpara high school and securing 1st in my batch.Then i have complete 12th standard from krishnath college school.i have completed my BSc in chemistry from krishnath college and my rank was 2nd in my college and 5th in kalyani university.Now i aam studying Master in school of chemistry , university of hyderabad
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How could the following compounds be synthesized, starting with a hydrocarbon that has the same number of carbon atoms as the desired product? a. b. CH3CH2CH2CH2OH c. d. CH3CH2CH2CH2CH2Br CH...
-
How could the following compounds be prepared from a carbonyl compound with no carbon-carbon double bonds? a. b. CH CH-CHCCH2CH2CH3 C CH-CH2 CH3
-
Show how the following compounds could be prepared, starting with 3 cyanocyclohexanone a. b. CH2CH2CCH
-
Louise Kalbe drew a check in the amount of $7,260 payable to the order of cash on her account at the Pulaski State Bank. The check was lost or stolen, but Kalbe did not report this to the bank, nor...
-
Explain how signal amplification is achieved in enzymelinked immunosorbent assays.
-
A group of twenty community college faculty instructors in Minnesota refused to join the Minnesota Community College Faculty Association. Under state law, faculty unions were given the exclusive...
-
Thickness of dust on solar cells. The performance of a solar cell can deteriorate when atmospheric dust accumulates on the solar panel surface. In the International Journal of Energy and...
-
Turtle, a C corporation, has taxable income of $300,000 before paying salaries to the three equal shareholder-employees, Britney, Shania, and Alan. Turtle follows a policy of distributing all...
-
A company's 6% coupon rate, semiannual payment, $1,000 par value bond that matures in 25 years sells at a price of $579.94. The company's federal-plus-state tax rate is 30%. What is the firm's...
-
Katey is a California CPA practicing in California. Katey has been licensed in California for 20 years, all of those years in active status. She is a partner in the firm of Perry & Sherry, CPA's....
-
Name the following compounds: a. b. c. CH;CHCH,CH,CH,CH3 OH
-
Explain why the aldehydic hydrogen (the one attached to the carbonyl carbon) is not exchanged with deuterium. -OD CH;CH CD;CH D20
-
In Exercises show that is strictly monotonic on the given interval and therefore has an inverse function on that interval. f(x) = cos x, [0, ]
-
A retail product has the following standard costs established: Direct Material per unit - 2 pounds at $5 a pound Direct Labor per unit - 3 hours at $12 an hour Manufacturing Overhead - $5 per labor...
-
In a recent year, the Better Business Bureau settled 75% of complaints they received. (Source: USA Today, March 2, 2009) You have been hired by the Bureau to investigate complaints this year...
-
A 1200-ft equal tangent crest vertical curve is currently designed for 50 mph. A civil engineering student contends that 60 mph is safe in a van because of the higher driver's eye height. If all...
-
Required information [The following information applies to the questions displayed below.] Victory Company uses weighted-average process costing to account for its production costs. Conversion cost...
-
Finer, % 100 90 80 70 60 50 40 30 20 10 0 0.01 0.1 1 Size, mm L 10 100 Figure shows a grain size distribution curve of soil. Estimate the coefficient of curvature (Cc) of this soil.
-
If R = [0, 4] [1, 2], use a Riemann sum with m = 2, n = 3 to estimate the value of R (1 xy 2 ) dA. Take the sample points to be (a) The lower right corners (b) The upper left corners of the...
-
You are a Loan Officer with an Investment Bank. Today you need to set your lending parameters. They are: LTV: 55% 10 Year T-Bill: TBD Rate Markup: 300 Basis Points Term: 30 Years Amortization: 30...
-
Two isomers of 1,2-dichloroethene are known. One has a dipole moment of 2.4 D; the other has zero dipole moment. Draw the two isomers and explain why one has zero dipole moment. CHCl=CHCl 1,...
-
Draw the hydrogen bonding that takes place between: (a) Two molecules of ethanol. (b) Two molecules of propylamine. (c) A molecule of dimethyl ether and two molecules of water. (d) Two molecules of...
-
For each pair of compounds, circle the compound you expect to have the higher boiling point. Explain your reasoning. (a) (CH3)3C-C(CH3)3 and (CH3)2CH-CH2CH2-CH(CH3)2 (b) CH3(CH2)6CH3 and...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


