Name the following alkyl halides: (a) H3C Br Br | | || CH3CHCHCHCHCHCH3 CH3 (d) CHBr CH3CHCHCHCHCH3
Question:
Name the following alkyl halides:
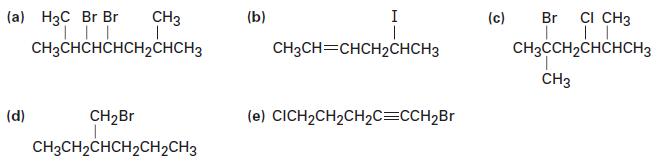
Transcribed Image Text:
(a) H3C Br Br | | || CH3CHCHCHCH₂CHCH3 CH3 (d) CH₂Br CH3CH₂CHCH₂CH₂CH3 (b) I CH3CH=CHCH2CHCH3 (e) CICH₂CH₂CH₂C=CCH₂Br (c) Br CI CH3 | || CH3CCH₂CHCHCH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a 1bromo22dimethylpropane b 2bromo3methyl pentane c 3bromo1chlorobutane d 2bromo...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give a JUPAC name for each of the following alkyl halides (yellow green =Cl): (b) (a)
-
Which of the following alkyl halides form a substitution product in an SN1 reaction that is different from the substitution product formed in an SN2 reaction? a. b. c. d. e. f. CH Br CH CHCHCHCH CHa...
-
Tell which of the following alkyl halides can give only one alkene, and which can give a mixture of alkenes, in the E2 reaction. (a) (b) CH,CH,CHCH,Br CH3
-
Discuss why the length of an OH bond obtained from X-ray diffraction experiments averages 85 pm whereas that obtained in neutron diffraction experiments averages 96 pm. Would you expect to see...
-
Describe how a manager who derives satisfaction from both income and shirking allocates a 10- hour day between these activities when paid an annual, fixed salary of $ 100,000. When this same manager...
-
Exercises relate to the function whose graph is sketched in Fig. 12. For what values of x does f (x) = 0? Y (2, 3) A 1 3 5 Figure 12 (7, -1) 9 y = f(x) X
-
Calories and salt in hot dogs. Is the correlation r for the data in Figure 14.11 near 1, clearly negative but not near 1, near 0, clearly positive but not near 1, or near 1? Explain your answer.
-
Brown & Co. issued seven-year bonds two years ago that can be called aft er two years. The bonds make semiannual coupon payments at a coupon rate of 7.875 percent. Each bond has a market value of...
-
QUESTION 2 HH Construction plc makes up its accounts to 31 March each year. The following details have been extracted in relation to two of its contracts as at 31 March 2005. Contract A Contract B...
-
Draw structures corresponding to the following names: (a) 2-Chloro-3,3-dimethyl hexane (b) 3,3-Dichloro-2-methyl hexane (c) 3-Bromo-3-ethyl pentane (d) 2-Bromo-5-chloro-3-methylhexane
-
Ignoring double-bond stereochemistry, what products would you expect from elimination reactions of the following alkyl halides? (a) Br CH3 | | CH3CH2CHCHCH3 (b) CH3 CI CH3 | | CH3CHCH2-C-CHCH3 CH3...
-
Based on what youve learned, pick one policy action undertaken by the U.S. government in response to the financial crisis. In a half-page essay, explain the policy action and the rationale behind the...
-
n1 = 20, n2 = 25, S = 607, H1: 1 2. In Exercises 710, compute S, S, and the value of the test statistic z. Then find the P-value for the specified alternate hypothesis and values of n1, n2, and S.
-
To determine whether traffic levels differ between the morning and evening rush hours, a traffic engineer counted the number of cars passing through a certain intersection during five-minute periods...
-
Macon Timber Company established a \(\$ 150\) petty cash fund on January 1, 2012. Required a. Is the establishment of the petty cash fund an asset source, use, or exchange transaction? b. Record the...
-
Following is a bank reconciliation for Holt's Sandwich Shop for May 31, 2012: Because of limited funds, Holt's employed only one accountant who was responsible for receiving cash, recording receipts...
-
For each of the following situations, fill in the blank with FIFO, LIFO, or weighted average. a. b. c. d. e. f. would produce the highest amount of net income in an inflationary environment. would...
-
A skateboarder starts up a 1.0-m-high, 30 ramp at a speed of 7.7 m/s. The skateboard wheels roll without friction. At the top, she leaves the ramp and sails through the air. How far from the end of...
-
Use nodal analysis to determine voltages v1, v2, and v3 in the circuit Fig. 3.76. Figure 3.76 4 S 3i, 2 A 4A
-
For each pair of the following compounds identify which compound would react more rapidly in an E1 reaction. a. b. CI .CI CI CI
-
Identify the stronger base: a) NaOH vs. H 2 O b) Sodium ethoxide vs. ethanol c) Ammonia vs. trimethylamine
-
(2S, 3S)-2-Bromo-3-phenylbutane undergoes an E2 reaction when treated with a strong base to produce (E)-2- phenyl-2-butene. Use Newman projections to explain the stereo-chemical outcome of this...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


