Give a JUPAC name for each of the following alkyl halides (yellow green =Cl): (b) (a)
Question:
Give a JUPAC name for each of the following alkyl halides (yellow green =Cl):
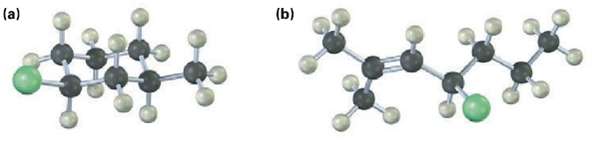
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a CH3 C CH3 CH3...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the IUPAC name for each of the following alkyl groups, and classify each one as primary, secondary, or tertiary: (a) CH3(CH2)10CH2-- (b) (c) --C(CH2CH3)3 (d) (e) (f) -CH2CH2CHCH2CH2CH3 CH2CH3...
-
Give the IUPAC name for each of the following alkyl groups, and classify each one as primary, secondary, or tertiary: (a) CH3 (CH2)10CH2 (b) CH2CH2CHCH2CH2CH3 W CH2CH3
-
Give a substitutive name for each of the following compounds. (a) CH3CHT-O-CH'CH'-OH (b)
-
The MRP gross requirements for Item A are shown here for the next 10 weeks. Lead time for A is three weeks and setup cost is $ 10. There is a carrying cost of $ 0.01 per unit per week. Beginning...
-
Consider the stylized example of Figure 7.3, in which there is a jump in the mean value. Using the same worksheet as a template, enter the exponential smoothing calculations for these periods. Assume...
-
Starting with the Fourier field equation in cylindrical coordinates, a. Reduce this equation to the applicable form for steady-state heat transfer in the θ direction. b. For the...
-
Rose, a minor, bought a new Buick Riviera from Sheehan Buick. Seven months later, while still a minor, he attempted to disaffirm the purchase. Sheehan Buick refused to accept the return of the car or...
-
On December 31, 2017, before the books were closed, the management and accountants of Madrasa Inc. made the following determinations about three pieces of equipment. 1. Equipment A was purchased...
-
How far discrimination of women and minorities happen in insurance industry career and how to resolve this problem?
-
Suppose the following items were taken from the December 31, 2017, assets section of the Concord Corporation balance sheet. (All dollarsa are in millions) Inventory $17,210 Patents $11.400 Notes...
-
Tell whether each of the following reactions is an oxidation, a reduction, orneither. (a) NABH4 H20 CH;CH- CH3CH2CH2OH (b) OH 1. BH3 2. NaOH, H202
-
Show the product(s) of reaction of the following alkenes withNBS: (b) (a)
-
Define done of lymphocytes.
-
A flat sheet is in the shape of a rectangle with sides of lengths 0.400 m and 0.600 m. The sheet is immersed in a uniform electric field of magnitude 85.0 N/C that is directed at 20 from the plane of...
-
You are a new BCBA working for a therapeutic day school that serves individuals with disabilities from ages 5 through 21. Each classroom has 1 special education teacher, 1 behavior therapist (BT) and...
-
Mountain Sports, Inc., is a retailer that has engaged you to assist in the preparation of its financial statements at December 31, 2021. Following are the correct adjusted account balances, in...
-
(a) sint 1-cost (b) Please note each question has 4 options: (a), (b), (c) and (d). 1. Given x = t - sint and y = 1 - cost, then dy 1-cost t-sint = 1-cost sint sint cost-1 2. Given sin x cos y - 2 =...
-
Sometimes I forget a few items when I leave the house in the morning. For example, here are probabilities that I forget various pieces of footwear: left sock 0.2 right sock 0.1 left shoe 0.1 right...
-
The most successful orientation programs can best be characterized as trial by fire. A. True B. False
-
Sheldon and Leonard had a million-dollar idea. In order to make it happen, they have to do special research first. Only Kripke can help them in this matter. But Kripke is known to be the first-class...
-
The titration of 10.00 mL of HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution...
-
Construct a graph, similar to Figure 3-11, of the torsional energy of 3-methylpentane along the C2-C3 bond. Place C2 in front, represented by three bonds coming together in a Y shape, and C3 in back,...
-
The following names are all incorrect or incomplete, but they represent real structures. Draw each structure and name it correctly. (a) 2-ethylpentane (b) 3-isopropylhexane (c)...
-
Provide IUPAC names for the following compounds. (a) (CH3)2CHCH2CH3 (b) CH3-C(CH3)2-CH3 (c) (d) (e) (f) CH CH CHCH CH,CHCHCH le ' CH CH,CH, CH CH CH, CH CH C(CH CH,CH,CHCHCH, CH(CH2 CH CHCH,CH, CH)C...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...
-
Regarding Enron, this was a company that resulted in the creation of the Sarbanes-Oxley Act and many reforms to the accounting profession. Research the company and answer the following...

Study smarter with the SolutionInn App


