Name the following ethers by IUPAC rules: (a) (c) CH3 CH3 LL. CH3CHOCHCH3 Br LOCH 3 (b)
Question:
Name the following ethers by IUPAC rules:
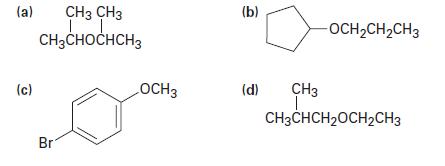
Transcribed Image Text:
(a) (c) CH3 CH3 LL. CH3CHOCHCH3 Br LOCH 3 (b) (d) -OCH₂CH₂CH3 CH3 T CH3CHCH₂OCH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a CH3OCHCH32 This ether is named methoxydimethylmethane accordi...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write equations for the synthesis of the following ethers by the Williamson method: a. b. (CH3)3COCH3 c. C6H5CH2OC(CH3)3 OCH3
-
Name the following molecules according to the IUPAC system of nomenclature. (a) (b) (c) (d) (e) CH3CH(CH3)CH(CH3)CH(CH3)CH(CH3)2 (f) CH3CH2CHCH3 CH CH H3C CH3 CHCHCH2CH3 EH CHCH,CH.CCH,CH.CH CH,...
-
Name the following molecules according to the IUPAC nomenclature system. (a) (b) (c) (d) (e) (f) CH32 Cl CH3 Br
-
True or False The slope of the line 2y = 3x + 5 is 3.
-
Use the estimated elasticities in Table 7 4 to calculate the Rothschild index for each industry. Based on these calculations, which industry most closely resembles perfect competition? Which industry...
-
Visit joomla.com to learn more about this open source content management system. What types of content does i t manage? Who uses Joomla, and what are its advantages? Visit two sites that use Joomla...
-
From the following particulars, prepare operating cost sheet. Total units generated 20,00,000 kWh. Operating labour Rs 50,000 Repairs Rs 50,000 Lubricants Rs 40,000 Plant supervision Rs 30,000...
-
Westbrook Co. predicts that it will use 225,000 gallons of material during the year. The material is expected to cost $10 per gallon. It anticipates that it will cost $40 to place each order. The...
-
Consider the following financial information about stock NEA: High Growth Stage (2 years) Stable Growth Stage g 10% g 3% EBIT(1-T) $500m WACC 10% CAPEX -$350m Dep. $100m NCWC -$200m Market Value of...
-
What product would you expect from the S N 1 reaction of (S)-3-methyloctan-3-ol [(S)-3-hydroxy-3-methyloctane] with HBr? Show the stereochemistry of both starting material and product.
-
What effect would the following changes have on the rate of the S N 1 reaction of tert-butyl alcohol with HBr? (a) The HBr concentration is tripled. (b) The HBr concentration is halved, and the...
-
Determine the magnitude of the resultant force F R and its direction, measured clockwise from the positive u axis. 70 45 F = 500 N V 30 U F = 300 N
-
Design a clocked D flip-flop, using a modified ECL circuit design, such that the output becomes valid on the negative-going edge of the clock signal.
-
An L2 steel strap having a thickness of 0.125 in. and a width of \(2 \mathrm{in}\). is bent into a circular arc of radius \(600 \mathrm{in}\). Determine the maximum bending stress in the strap.
-
Cars traveling from Canada to the United States through the Thousand Islands Border Crossing must stop for US Customs and Immigration. During the stop, each passenger in the car must present a...
-
Gasoline is pumped through a 2 in. sch 40 pipeline upward into an elevated storage tank at $60^{\circ} \mathrm{F}$. An orifice meter is mounted in a vertical section of the line, which uses a DP cell...
-
Change the recurring costs in Problem and Exercise 3 to $40,000 and redo the analysis. Problem and Exercise 3 Assume you are put in charge of launching a new website for a local nonprofit...
-
A blood sample is found to contain 8.6 mg / dL of Ca. How many atoms of Ca are present in 8.6 mg? The atomic weight of Ca is 40.08 amu.
-
You deposit $10,000 in a savings account that earns 7.5% simple interest per year. What is the minimum number of years you must wait to double your balance? Suppose instead that you deposit the...
-
Draw a mechanism for each of the following E1 processes: a. b. c. d. H,SO, Heat Br ETOH, Heat
-
Identify the pattern for each mechanism in Problem 8.34. For example the pattern for Problem 8.34a is: This mechanism is comprised of a proton transfer followed by the two core steps of an E1 process...
-
Identify which of the following methods is more efficient for producing 3,3 dimethylcyclohexene. Explain your choice. Br NaOEt EEOH OH H,SO, Heat
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


