Identify which of the following methods is more efficient for producing 3,3 dimethylcyclohexene. Explain your choice. Br
Question:
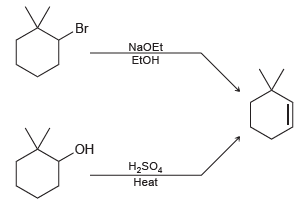
Transcribed Image Text:
Br NaOEt EEOH OH H,SO, Heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 86% (15 reviews)
The first method is more efficient because it employs a strong ...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
324+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify which of the following monomers would be most reactive toward anionic polymerization: CH3 OAc CN
-
Identify which of the following monomers would be most reactive toward cationic polymerization. OAc CN CI
-
Identify which of the following functions are eigen functions of the operator d/dx: (a) D2/dx2, (b) Cos kx, (c) K, (d) Kx, (e) e-ax2. Give the corresponding eigen value where appropriate.
-
Discuss the interface between Purchasing and Supply Management and Logistics Management specifically with respect to selection of a third party logistics provider, analysis of the total cost of...
-
Prepare an analysis of the property rights, risks, and benefits of each in a minimum of, excluding the title and reference page, including the following: Decide what actions a manager in your...
-
In Exercises find the points of horizontal and vertical tangency (if any) to the polar curve. r = 1 - cos
-
Define social media and describe how they differ from traditional advertising media.
-
Mike Samson is a college football coach making a base salary of $652,800 a year ($54,400 per month). Employers are required to withhold a 6.2% Social Security tax up to a maximum base amount and a...
-
Income Statement, Retained Earnings Statement, and Balance Sheet The amounts of the assets and liabilities of Glacier Travel Service at September 30, 2016, the end of the current year, and its...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Identify the pattern for each mechanism in Problem 8.34. For example the pattern for Problem 8.34a is: This mechanism is comprised of a proton transfer followed by the two core steps of an E1 process...
-
Calculate H and S if the temperature of 1.75 moles of Hg(l) is increased from 0.00 o C to 75.0 o C at 1 bar. Over this temperature range, C P,m = (J K -1 mol -1 ) 30.093 4.944 10 -3 T/K.
-
How has the role of the IMF come under scrutiny in the recent discussion of reforms in the international financial architecture?
-
The following table contains the monthly operating costs of a company. Salary is not included. Determine the variance and standard deviation of the costs. Enero Febrero Marzo Abril Mayo Junio Julio...
-
Becker & Smith, CPAs, performs a financial statement review for BAM Markets ( BAM ) . Caroline, the manager on the job, learns that Don, a member of the review team, violated the independence rules....
-
Presented here are selected transactions for Sheridan Inc. during August of the current year. Sheridan uses a perpetual inventory system. It estimates a return rate of 10%, based on past experience....
-
. Complete both parts (a) and (b) below. ). In1 (a) Let X11, X12, ..., X be a random sample of size n from a population with mean and variance . Let X21, X22,..., X2n2 be a random sample of size n...
-
41. Let S be the cone z = x + y, z 2, oriented with outward unit normal. Use Stokes' theorem to evaluate the flux integral for the vector field SJ (V x F). ndS F(x, y, z) = (x y)i + 2zj + xk. -
-
Factor out the greatest common factor. 7x 3 + 35x 4 - 14x 5
-
Find the cross product a x b and verify that it is orthogonal to both a and b. a = (t, 1, 1/t), b = (t 2 , t 2 , 1)
-
What product(s) would you expect from the reaction of 1-methylcyclohexene with NBS? Would you use this reaction as part of asynthesis? CH NBS CCI4
-
How would you prepare the following compounds, starting with cyclopentane and any other reagents needed? (a) Chloro cyclopentane (b) Methylcyclopentane (c) 3-Bromocyclopentene (d) Cyclopentanol (e)...
-
Predict the product(s) of the followingreactions: SoCI. (b) CCH2H2CH2 () HBr Ether (d) (c) NBS PBr3 Ether Mg H20 A? (e) CH3CH2CHBrCH3 ? Ether Cul Li A? ? (f) CH3CH2CH2CH2BR Pentane Ether (g)...
-
ABC company makes turbo-encabulators, customized to satisfy each customers order. They split overhead into five pools, each with its own activity driver (direct labor for manufacturing, direct labor...
-
Variable manufacturing overhead becomes part of a unit's cost when variable costing is used.Group of answer choicesTrueFalse
-
Santa Fe Corporation has computed the following unit costs for the year just ended:Direct Material used $23Direct Labor $18Fixed selling and administrative cost $18Variable manufacturing overhead...

Study smarter with the SolutionInn App


