Identify which of the following monomers would be most reactive toward anionic polymerization: CH3 OAc CN
Question:
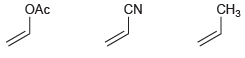
Transcribed Image Text:
CH3 OAc CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 65% (20 reviews)
least r...View the full answer

Answered By

Ashish Buragohain
For me tutoring is very good.It is a learning curve for me as well as for students.Through my time in tutoring i have learn many things which helped me in my profession.I have also learnt that not only we have to be good at explaining but also at giving encouragement and motivating learners.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify which of the following monomers would be most reactive toward cationic polymerization. OAc CN CI
-
Which of the following monomers might you expect would lead to a conducting polymer? a. b. H2C==CHCH3 --,
-
Which of the following monomers might you expect to lead to a conducting polymer? a. CH3CH==CHCH3 b. CH CC CH
-
(a) the maximum shear stress magnitude T1 in aluminum tube (1). (b) the maximum shear stress magnitude T2 in brass shaft segment (2). (c) the maximum shear stress magnitude T3 in brass shaft segment...
-
Consider the following partially completed income statements for merchandising companies and compute the missing amounts: Smith, Inc. Allen, Inc. $ 101,000 Net Sales Revenue (d) Cost of Goods Sold:...
-
What volume of oxygen at 24C and 0.88 atm is needed to completely react via combustion with 45 g of methane gas?
-
Hourly Wages A labor organization estimates that the median hourly wage of podiatrists is at least $55.89. In a random sample of 23 podiatrists, 17 are paid less than $55.89 per hour, 5 are paid more...
-
Comparative data from the balance sheet of BlackBerry Limited are shown below. Instructions (a) Using horizontal analysis, calculate the percentage change between 2014 and 2015. (b) Using vertical...
-
The TP ' s sole proprietorship had the following revenues and expenses during 2 0 2 3 : Sales Revenue Sales Returns Cost of Goods Sold Interest Expense on business loan Car / Truck Expense ( repairs...
-
In November 2015, John Wells, a customer service representative of Bayfield Mud Company, was summoned to the Houston warehouse of Wet-Land Drilling, Inc., to inspect three boxcars of mud treating...
-
Nitroethylene undergoes anionic polymerization so rapidly that it is difficult to isolate nitroethylene without it polymerizing. Explain.
-
Draw a region of an alternating copolymer constructed from vinyl chloride and ethylene.
-
When is the market for real money balances in equilibrium? If the Fed engages in open market sales, what happens to the supply of real balances?
-
How have your organizations performed relative to improving healthcare quality and meeting the required standards (Medicare metrics) for value-based purchasing initiatives?
-
/ Precalculus Algebra Problem. 1: Consider the function f(x)=-5x5 + +-4. How many terms in f(x) are not monomials? Problem. 2: Consider the function f(x)=-3x-4x - 3x + 12. How many terms in f(x) are...
-
D 0
-
What NaCl concentration results when 279 mL of a 0.680 M NaCl solution is mixed with 462 mL of a 0.450 M NaCl solution? concentration: M
-
Use JavaFX's shape's classes from javafx.scene.shape package to complete the following questions (Hint: CANNOT use any Gaphics or Graphics2D classes from java.awt packages): DO not write the whole...
-
Evaluate the following integrals. A sketch of the region of integration may be useful. LITE -21 J1 xy Z -dz dx dy
-
Use nodal analysis to determine voltages v1, v2, and v3 in the circuit Fig. 3.76. Figure 3.76 4 S 3i, 2 A 4A
-
Show how to synthesize the following amines from the indicated starting materials by acylation-reduction. (a) N-butylpiperidine from piperidine (b) N-benzylaniline from aniline
-
Addition of one equivalent of ammonia to 1-bromoheptane gives a mixture of heptan-1-amine, some dialkylamine, some trialkylamine, and even some tetraalkylammonium bromide. (a) Give a mechanism to...
-
Show how Gabriel syntheses might be used to prepare the following amines. (a) Benzylamine (b) hexan-1-amine (c) g-aminobutyric acid
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


