Predict the product(s) of the following transformations: (a) (c) CHOH Periodinane CH3 HC=CHCHCHCOCH 1. LIAIH4 2.
Question:
Predict the product(s) of the following transformations: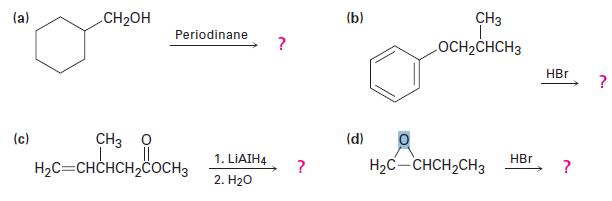
Transcribed Image Text:
(a) (c) CH₂OH Periodinane CH3 Ọ H₂C=CHCHCH₂COCH 1. LIAIH4 2. H₂O ? ? (b) (d) CH3 I OCH₂CHCH3 H₂C-CHCH₂CH3 HBr HBr ? ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a CH2OH Periodinane The reaction with periodinane usually results in the oxidation of the alcohol gr...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major organic product of each of the following reactions. In spite of the structural complexity of some of the starting materials, the functional group transformations are all of the type...
-
Predict the major organic product of each of the following reactions. In spite of the structural complexity of some of the starting materials, the functional group transformations are all of the type...
-
The following reaction does not produce the product shown. (a) Predict the major product from the conditions shown above, and write a detailed mechanism for its formation. (b) What reaction...
-
Which of the following variables was controlled in Experiment 1? F. Amount of yeast G. Percent of molasses H. Percent of sucrose J. Carbon dioxide levels Experiment 1 Since yeast needs sucrose to...
-
The elasticity of demand for a firms product is -2.5 and its advertising elasticity of demand is 2.0. a. Determine the firms optimal advertising-to-sales ratio. b. If the firms revenues are $40,000,...
-
What other strategic actions w ill GameStop need to take to protect its business? With more than 6,600 stores throughout the United States and 14 other countries, GameStop's management team wants to...
-
XY & Co. owns a fleet of 10 trucks each costing Rs 60,000. The company has employed one manager to whom it pays Rs 450 p.m., an accountant who gets Rs 250 p.m. and a peon who gets Rs 100 p.m. The...
-
Sometimes, the root cause of a variance requires that variance analysis be performed in both hours and dollars. It is possible that calculating the variances in both hours and dollars is the only way...
-
Use the following tables to calculate the present value of a $516,000 5%, 6-year bond that pays $25,800 Interest annually, if the market rate of interest is 6%. Round to the nearest dollar Present...
-
Show how you could prepare the following substances from cyclohexanol: (a) (b) -Br (c) (d)
-
What effect on the rate of an E1 reaction of 2-chloro-2-methylpropane would you expect if the concentration of the alkyl halide tripled?
-
Green Lawns, Inc., performs adjusting entries every month, but closes its accounts only at year-end. The company's year-end adjusted trial balance dated December 31, 2015, was: a. Prepare an income...
-
Convert the following information into: a) a semantic net b) a frame-based representation A Ford is a type of car. Bob owns two cars. Bob parks his car at home.His house is in California, which is a...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
A group of four-month-old hogs has an average weight of 170 pounds. The average weight of selected breeders is 185 pounds. If the heritability of weight is 40%, what is the expected average weight of...
-
MgO prevents premature evaporation of Al in a furnace by maintaining the aluminum as Al2O3. Another type of matrix modifier prevents loss of signal from the atom X that readily forms the molecular...
-
Predict the major and minor product for each of the following E2 reactions: a. b. c. d. e. f. ? NaOEt I Br NaOEt,
-
Draw an alkyl halide that will produce only one alkene upon treatment with a strong base.
-
Draw an alkyl halide that will produce exactly two stereo-isomeric alkenes upon treatment with a strong base.
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


