Show how you could prepare the following substances from cyclohexanol: (a) (b) -Br (c) (d)
Question:
Show how you could prepare the following substances from cyclohexanol: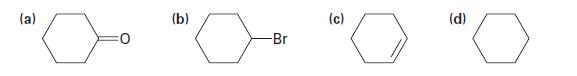
Transcribed Image Text:
(a) (b) -Br (c) (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
To prepare the following substances from cyclohexanol we need to perform specific chemical reactions to transform cyclohexanol into the desired produc...View the full answer

Answered By

Saleem Abbas
Have worked in academic writing for an a years as my part-time job.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how you could prepare the following substances from propan-1-ol: (a) O || CH3CHCH (b) O CH3CHCOH (c) CH3CHCHO Na+ (d) CH3CHCHCl
-
Show how you could prepare each of the following compounds. Use the starting material indicated along with ethyl acetoacetate or diethyl malonate and any necessary inorganic reagents. Assume also...
-
Show how you could prepare each of the following compounds from cyclopentanone, D2O, and any necessary organic or inorganic reagents. H OH
-
Why is the number of equivalent units for materials only sometimes equal to the equivalent units for conversion?
-
A monopolists inverse demand function is P = 150 - 3Q. The company produces output at two facilities; the marginal cost of producing at facility 1 is MC1 (Q1) = 6Q1, and the marginal cost of...
-
You borrow $37,120 to attend college and must repay your loan at 4.065% APR... a. How many months will it take to repay this debt if you choose to pay back $500/month? b. In this scenario, what is...
-
From the following, data relating to vehicle X calculate the cost per running kilometre. Vehicle X Kilometres run (annual) 15,000 Tons per km (average) 6 Cost of vehicle Rs 25,000 Road licence...
-
Presented below are selected accounts for Acevedo Company as reported in the worksheet at the end of May 2012. InstructionsComplete the worksheet by extending amounts reported in the adjusted trial...
-
Who holds the legal title to the trust property? Select one: Beneficiary Trustee Settlor Trustor
-
Draw structures corresponding to the following IUPAC names: (a) 2,3-Dichloro-4-methylhexane (b) 4-Bromo-4-ethyl-2-methylhexane (c) 3-Iodo-2,2,4,4-tetramethylpentane
-
Predict the product(s) of the following transformations: (a) (c) CHOH Periodinane CH3 HC=CHCHCHCOCH 1. LIAIH4 2. HO ? ? (b) (d) CH3 I OCHCHCH3 HC-CHCHCH3 HBr HBr ? ?
-
Three laws in the computation of sums are for any permutation p(k) of the set of integers k in the summation. (a) Explain why the above rules make sense when computing sums. To do that consider Let c...
-
Sketch the curves with equations given in question 3 parts a, b, c and d, labelling any stationary points with their coordinates. Data from Question 3 Find the coordinates of the points where the...
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 130 Q R T P S
-
Name any devices other than the three mentioned in Section 31. 1-battery, solar cell, and electric generator that can act as a power source in an electric circuit. Data from Section 31. 1...
-
A thick resistor and a thin resistor of the same length and material are connected in series, as shown in Figure 31. 29. Which resistor has \((a)\) the greater potential difference across it and...
-
Note that the amount of 25k that has already been spent on developing the website is not included in this analysis, as it represents a sunk cost. The decision rule for ARR is that a project should be...
-
A monopolist's demand function is P = 1624 - 4Q and its total cost function is TC = 22000 + 24q - 4Q squared + 1/3Q cubed Where q is output produced and sold. a. At what level of output and sales (Q)...
-
What is the maximum volume of 0.25 M sodium hypochlorite solution (NaOCl, laundry bleach) that can be prepared by dilution of 1.00 L of 0.80 M NaOCl?
-
Identify an alkyl halide that could be used to make the following alkene:
-
When menthyl chloride is treated with a strong base, only one elimination product is observed. Yet, when neomenthyl chloride is treated with a strong base, two elimination products are observed. Draw...
-
Predict which of the following two compounds will undergo an E2 reaction more rapidly: CI
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


