Show how you could prepare the following substances from propan-1-ol: (a) O || CH3CHCH (b) O CH3CHCOH
Question:
Show how you could prepare the following substances from propan-1-ol: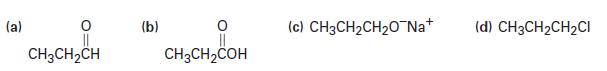
Transcribed Image Text:
(a) O || CH3CH₂CH (b) O CH3CH₂COH (c) CH3CH₂CH₂O Na+ (d) CH3CH₂CH₂Cl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
ANSWER a Propanal CH3CH2CHO To prepare propanal from propan1ol you need to perform an oxidation reaction One common method is to use an oxidizing agen...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how you could prepare the following substances from cyclohexanol: (a) (b) -Br (c) (d)
-
Show how you could prepare each of the following compounds. Use the starting material indicated along with ethyl acetoacetate or diethyl malonate and any necessary inorganic reagents. Assume also...
-
Show how you could prepare each of the following compounds from cyclopentanone, D2O, and any necessary organic or inorganic reagents. H OH
-
Tell whether the given side lengths of a triangle can represent a right triangle. 36, 48, and 60
-
You are the manager of a monopoly and your demand and the cost function are given by P = 300 3Q And C (Q) = 1,500 + 2Q2 Respectively. a. What price-quantity combination maximizes your firms profit?...
-
What were the early design approaches to managing information resources?
-
From the following data, find out the cost per room-day and the charge to the customers if the profit required is 20% on cost. Room accommodation available 50 double rooms 100 single rooms Each...
-
Frobisher Inc. (Frobisher) uses the lower of cost and NRV rule to value its inventory. Frobishers inventory on February 28, 2017 had a cost of $1,125,000 and a NRV of $1,035,000. Required: a. By how...
-
ABC Credit Co. has in its possession an undated note which provides that it is payable six months after the date on which it was issued. The note was issued on March 10, 2019, by Martin to Newman in...
-
What effect on the rate of an E1 reaction of 2-chloro-2-methylpropane would you expect if the concentration of the alkyl halide tripled?
-
What product would you expect from the S N 1 reaction of (S)-3-methyloctan-3-ol [(S)-3-hydroxy-3-methyloctane] with HBr? Show the stereochemistry of both starting material and product.
-
Cullen Construction Company changed from the completed-contract to the percentage-of-completion method of accounting for long-term construction contracts during 2008. For tax purposes, the company...
-
Recall from Case 1.2 that Auto Concepts is a new division of a large automobile manufacturer that has been slowly losing market share to its competitors. Auto Concepts was created to reclaim the...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a diverging lens. \((b)\) Is the image real or virtual? (c) Is it upright or...
-
Show that the ray exiting the block in Figure P33.53 is parallel to the ray entering the block. Data from Figure P33.53
-
The element is subjected to the state of stress shown. If the material is machine steel having a yield stress of \(\sigma_{Y}=750 \mathrm{MPa}\), determine the factor of safety with respect to...
-
Determine the vertical displacement of the ring at point \(B\). \(E I\) is constant. B P A
-
According to the graph below, how frequently do objects the size of comet Temple 1 strike Earth? a. Once in Earth's history b. About once every hundred million years c. About once every million years...
-
Suppose the concentration of glucose inside a cell is 0.1 mm and the cell is suspended in a glucose solution of 0.01 mm. a. What would be the free energy change involved in transporting 10-o mole of...
-
Draw the carbocation intermediate generated when each of the following substrates is treated with sulfuric acid: a.
-
Identify the major and minor products for each of the following E1 reactions: a. b. c. d. H,SO. eat ETOH Heat Br
-
Identify two different starting alcohols that could be used to make 1-methylcyclohexene. Then determine which alcohol would be expected to react more rapidly under acidic conditions. Explain your...
-
BE13.2 (LO 1), AP An inexperienced accountant for Silva Corporation showed the following in the income statement: net income \$337,500 and unrealized gain on availablefor-sale securities (before...
-
A start - up company is seeking $ 5 m for its Series A investment round. The start - up is expected to grow to $ 1 0 0 M in sales and $ 1 0 M in profit by year 5 . Comparable firms in the industry...
-
Here are the cash flows for a project under consideration: C 0 C 1 C 2 $8,010 +$5,940 +$20,160 a. Calculate the projects net present value for discount rates of 0, 50%, and 100%. (Round your answers...

Study smarter with the SolutionInn App


