Show how the following molecule can be prepared from a carbonyl compound and an amine (blue=N):
Question:
Show how the following molecule can be prepared from a carbonyl compound and an amine (blue=N):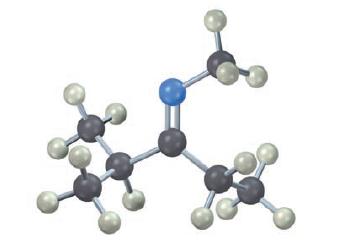
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
The given molecule is an imine which can be prepared from a carbonyl compound and an ...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How could the following compounds be prepared from a carbonyl compound with no carbon-carbon double bonds? a. b. CH CH-CHCCH2CH2CH3 C CH-CH2 CH3
-
The following molecule can be prepared by reaction between a primary amine and a dihalide. Identify the two reactants, and write the reaction.
-
a. Write the mechanism for the following reactions: 1. The acid-catalyzed hydrolysis of an imine to a carbonyl compound and a primary amine 2. The acid-catalyzed hydrolysis of an enamine to a...
-
Factor each trinomial. 4(m 5)2 4(m 5) 15
-
A large Coca- Cola vendor recently hired some economic analysts to assess the effect of a price increase in its 16-ounce bottles from $ 1.00 to $ 2.00. The analysts determined that, on average, the...
-
Simplify each of the given expressions. (a) j 21 (b) (j) 21
-
Service costing is applicable in canteens.
-
On January 1, 2014, the Vasquez Company ledger shows Equipment $32,000 and Accumulated Depreciation-Equipment $9,000. The depreciation resulted from using the straight-line method with a useful life...
-
Which of the following strategies would best be used to reduce the break-even point? a. Increase in both variable cost per unit and contribution margin per unit b. Increase in variable cost per unit...
-
The herbicide 2,4,5-T (2,4,5-trichlorophenoxyacetic acid) can be prepared by heating a mixture of 2,4,5-trichlorophenol and ClCH 2 CO 2 H with NaOH. Show the mechanism of the reaction.
-
How might you prepare the following alcohols from an aldehyde or ketone? Show all possibilities. (a) H OH (b) CHCHOH (c) HO CH3 CH3
-
Kwan Corp. started operations on January 1, 2011. It is now December 31, 2011: the end of the fiscal year. The part-time bookkeeper needs your help to analyze the following three transactions: a. On...
-
Once the largest professional services firm in the world and arguably the most respected, Arthur Andersen LLP (AA) has disappeared. The Big 5 accounting firms are now the Big 4. Why did this happen?...
-
Fill in the Blank. Piezoelectric transducers generate electrical ______________ when subjected to mechanical stress.
-
A clockwise variable torque is applied to a flywheel at time \(t=0\) causing its clockwise angular acceleration to decrease linearly with angular displacement \(\theta\) during 20 revolutions of the...
-
With neat block diagram, explain open-loop and closed-loop control systems.
-
Market-Tech, a market research firm, had the following transactions in June, its first month of operations. 1 \( \mathrm{~J}\). Witson invested \(\$ 28,000\) of personal funds in the firm in exchange...
-
Fiona requires a minimum level of consumption, a threshold, to derive additional utility: U(X, Z) is 0 if X + Z ( 5 and is X + Z otherwise. Draw Fiona's indifference curves. Which of our preference...
-
Estimate a range for the optimal objective value for the following LPs: (a) Minimize z = 5x1 + 2x2 Subject to X1 - x2 3 2x1 + 3x2 5 X1, x2 0 (b) Maximize z = x1 + 5x2 + 3x3 Subject to X1 + 2x2 +...
-
A compound with molecular formula C 10 H 10 O 4 produces a 1 H NMR spectrum that exhibits only two signals, both singlets. One signal appears at 3.9 ppm with a relative integration value of 79. The...
-
For each of the following compounds, predict the number of signals and location of each signal in a 13 C NMR spectrum: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) H.
-
Compare the following two constitutional isomers. The 13 C NMR spectrum of the first compound exhibits five signals, while the second compound exhibits six signals. Explain. .
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


