How might you prepare the following alcohols from an aldehyde or ketone? Show all possibilities. (a) H
Question:
How might you prepare the following alcohols from an aldehyde or ketone? Show all possibilities.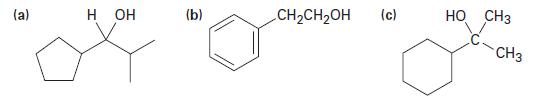
Transcribed Image Text:
(a) H OH (b) CH₂CH₂OH (c) HO CH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
To prepare the specified alcohols from an aldehyde or ketone youll need to use reduction reactions T...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How might you use a Grignard reaction of an aldehyde or ketone to prepare the following molecule (red=O)?
-
Treatment of an aldehyde or ketone with cyanide ion (: C N), followed by proton-ation of the tetrahedral alkoxide ion intermediate, gives a cyanohydrins. Show the structure of the cyanohydrins...
-
Unlike a phosphonium ylide, which reacts with an aldehyde or ketone to form an alkene, a sulfonium ylide reacts with an aldehyde or ketone to form an epoxide. Explain why one ylide forms an alkene,...
-
A garden has an area of 320 ft 2 . Its length is 4 ft more than its width. What are the dimensions of the garden? X x +4 X
-
Recently, an Internet service provider ( ISP) in the UK implemented a no- strings US- style flat-rate plan whereby its commercial subscribers can send and receive unlimited volume (measured in...
-
The force F that acts on a pendulum bob is proportional to the mass m of the bob and the sine of the angle the pendulum makes with the vertical. If F = 0.120 N for m = 0.350 kg and = 2.00, find F...
-
The unit used in service costing is simple.
-
The two blocks in Fig. 4.39 are connected by a heavy uniform rope with a mass of 4.00 kg. An upward force of 200 N is applied as shown. (a) Draw three free-body diagrams, one for the 6.00-kg block,...
-
What is the difference between an angel investor and a venture capitalist? What event do these investors want to see happen? Why?
-
Show how the following molecule can be prepared from a carbonyl compound and an amine (blue=N):
-
Show how you might carry out the following transformation. (A protection step is needed.) OH HOCHSCHICHYLOCH, 2 HECHICHICHICCHS ? HCCHCHCHCOCH3 HCCHCHCHCCH3 CH3
-
View the video, ''HACCP Training for Food Handlers," https://www.youtube.com/watch?v=mE-q9W4jqQg. At what temperature range can food become unsafe?
-
Arrow Company processes a food seasoning powder through its Compounding and Packaging departments. In the Compounding Department, direct materials are added at the beginning of the process, and...
-
The 2017 financial statements of LVMH Moet Hennessey Louis Vuitton S.A. are presented in Appendix C at the end of this book. LVMH is a Paris-based holding company and one of the world's largest and...
-
Repeat Problem 10.E1, except design a packed column using 1-in. metal Pall rings. Do the calculations at the top of the column. Approximate HETP for ethanol-water is \(0.366 \mathrm{~m}\). At...
-
We are separating an ethanol-water mixture in a column operating at atmospheric pressure with a total condenser and a partial reboiler. Constant molal overflow (CMO) can be assumed, and reflux is a...
-
Corporate Social Responsibility Problem The Global Reporting Initiative (GRI) is a networkbased organization that has pioneered the development of the world's most widely used sustainability...
-
Sofia will consume hot dogs only with whipped cream. Show her preference map. What is her utility function?
-
Show, if u(x, y) and v(x, y) are harmonic functions, that u + v must be a harmonic function but that uv need not be a harmonic function. Is e"e" a harmonic function?
-
Draw the structure of a compound with molecular formula C 8 H 10 that exhibits five signals in its 13 C NMR spectrum, four of which appear between 100 and 150 ppm.
-
Determine the structure of a compound with molecular formula C 5 H 10 O that exhibits the following broadband-decoupled and DEPT-135 spectra. The DEPT-90 spectrum has no signals. Broadband-decoupled...
-
Determine the structure of an alcohol with molecular formula C 5 H 12 O that exhibits the following signals in its 13 C NMR spectra: (a) Broadband decoupled: 73.8 , 29.1 , and 9.5 (b) DEPT-90: 73.8 ...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


