Show the products of the following reactions: (a) (c) CH3CHCHNH CH3CH2CNH2 CH3Br 1. LiAlH4 2. HO ?
Question:
Show the products of the following reactions: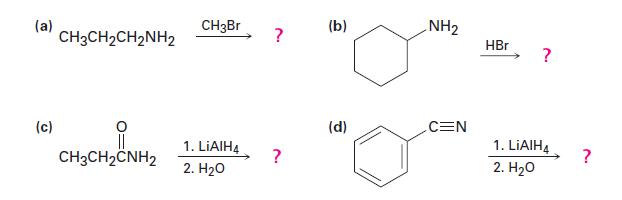
Transcribed Image Text:
(a) (c) CH3CH₂CH₂NH₂ CH3CH2CNH2 CH3Br 1. LiAlH4 2. H₂O ? ? (b) (d) NH₂ CEN HBr ? 1. LIAIH4 2. H₂O ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
A Amines RNH2 react alkyl halidesCH3Br to give ammonium salts R3NBr If we use ...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show the products of the reactions of these carboxylic acids with PB3/Br2 before and after hydrolysis. (a) Pentanoic acid (b) Phenylacetic acid (c) Succinic acid (d) Oxalic acid
-
Show how you would use Suzuki reactions to synthesize these products from the indicated starting materials. You may use any additional reagents you need. (a) (b) Br
-
Show how Diels-Alder reactions might be used to synthesize the following compounds. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH 3COOCH CH3 CN CN CI CI Cl Cl Cl CI CI C chlordane CI CI Cl Cl CI C aldrin CN...
-
In Exercises 8486, use a graphing utility to graph f and g in the same [-8, 8, 1] by [-5, 5, 1] viewing rectangle. In addition, graph the line y = x and visually determine if f and g are inverses....
-
In an effort to make the company more competitive, Fast-Guard, Inc., incurred significant expenses related to a reduction in the number of employees, consolidation of offices and facilities, and...
-
Verify the given moment(s) of inertia and find x and y. Assume that each lamina has a density of = 1 gram per square centimeter. Ellipse b a 10=nab(a + b) X
-
Real wages (optional). In one of the many reports on stagnant incomes in the United States, we read this: Practically every income group faced a decline in real wages during the 1980s. However,...
-
The following tabulations are actual sales of units for six months and a starting forecast in January. a. Calculate forecasts for the remaining five months using simple exponential smoothing with a =...
-
A Data Table B Physical 1 Transferred-In Costs D Direct Materials Units (tons) Conversion Costs 1001$ OS 30,000 75,000 $ 100% 0% 60% 185 2 Work in process, beginning inventory (June 1) 3 Degree of...
-
How could you prepare the following amines from ammonia and appropriate alkyl halides? (a) Triethylamine (b) Tetramethylammonium bromide
-
How might you prepare the following amines from ammonia and any alkyl halides needed? (a) CH3CHCHCHCHCHNH CHNH2 (c) (b) (CH3)4N+ I- (d) NHCH3
-
The following selected events occurred for Orwell Company during the first quarter of 2019: Jan. 11 A motor breaks on a machine and is replaced for $2,400. This replacement was expected when the...
-
REQUIRED: Cost of production report under the following assumptions: Lost units - normal, discovered at the beginning Lost units - normal, discovered at the end Lost units - abnormal, discovered when...
-
ABC, Inc., manufactures only two products: Gadget A and Gadget B. The firm uses a single, plant wide overhead rate based on direct-labor hours. Production and product-costing data are as follows:...
-
.Jean Saburit has gone over the financial statements for Saburit Parts, Inc. The income statement has been prepared on an absorption costing basis and Saburit would like to have the statement revised...
-
When a constant force is applied to an object, the acceleration of the object varies inversely with its mass. When a certain constant force acts upon an object with mass 2 kg, the acceleration of the...
-
Use the following for all 3 circuits. V1 = 9.0 V, V = 12.0 V R = 2.0 ohms, R = 4.0 ohms, R3 = 6.0 ohms, R4 = 8.0 ohms C1 = 3.0 C = 3.0 (a) Find I in circuit A (b) Find I1 in circuit B R w R3 V R R4...
-
Suppose that the inverse market demand for an upcoming Bruce Springsteen concert at Philadelphia's 20,000-seat Wachovia Center is p = 1,000 - 0.04Q. Mr. Springsteen is concerned about the well-being...
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
The following compound is optically inactive. Explain why.
-
Identify the reagent you would use to accomplish each of the following transformations: (a) Cyclobutanol bromocyclobutane (b) tert-Butanol tert-butyl chloride (c) Ethyl chloride ethanol
-
How many different alkenes will produce 2, 4-dimethylpentane upon hydrogenation? Draw them.
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


