Question: Shown below is the final step in a synthesis of an important perfume constituent, cis-jasmone. Which reagents would you choose to carry out this last
Shown below is the final step in a synthesis of an important perfume constituent, cis-jasmone. Which reagents would you choose to carry out this last step?
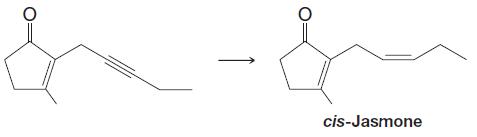
cis-Jasmone
Step by Step Solution
3.22 Rating (166 Votes )
There are 3 Steps involved in it
This reaction can be carried out using LindlarsCatalyst PdCaCO3PbOAc3Quinoline which ... View full answer

Get step-by-step solutions from verified subject matter experts


