Use the bond-dissociation enthalpies in Table 4-2 to calculate the heats of reaction for the two possible
Question:
Use the bond-dissociation enthalpies in Table 4-2 to calculate the heats of reaction for the two possible first propagation steps in the chlorination of isobutane. Use this information to draw a reaction-energy diagram like Figure 4-8, comparing the activation energies for formation of the two radicals.
Table 4-2
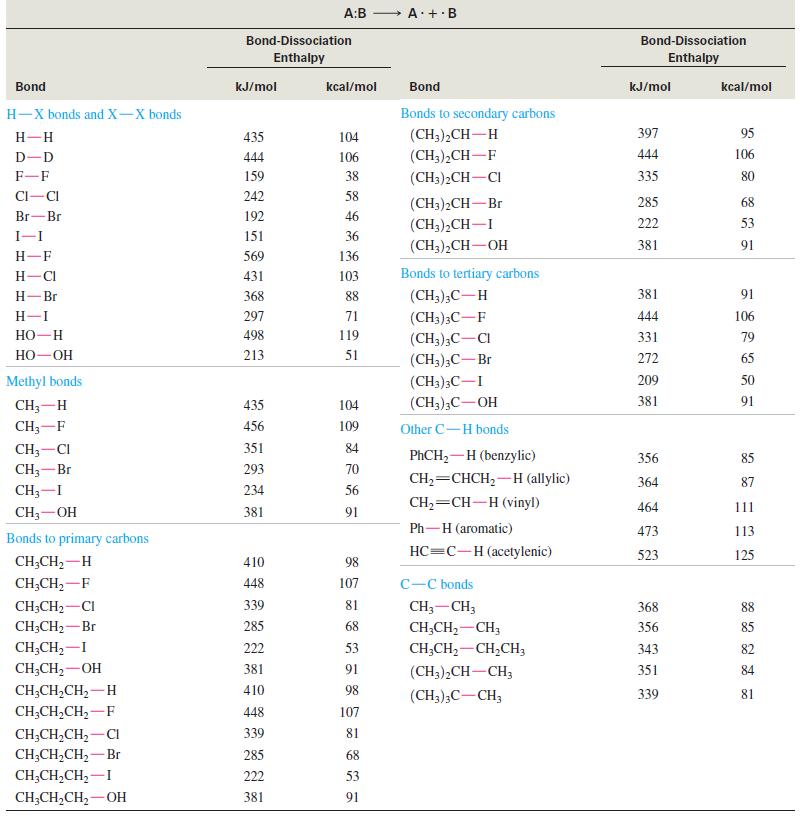
Transcribed Image Text:
A:B → A.+·B Bond-Dissociation Bond-Dissociation Enthalpy Enthalpy Bond kJ/mol kcal/mol Bond kJ/mol kcal/mol H-X bonds and X–X bonds Bonds to secondary carbons 397 (CH3),CH-H (CH3),CH-F H-H 435 104 95 D-D 444 106 444 106 F-F 159 38 (CH3)2CH-CI 335 80 Cl-CI 242 58 (CH3)2CH- Br 285 68 Br -Br 192 46 (CH3)2CH-I (CH3),CH-OH 222 53 I-I 151 36 381 91 H-F 569 136 431 103 Bonds to tertiary carbons 1D-H (CH3),C-H (CH3)3C-F (CH3);C-CI (CH3);C-Br H-Br 368 88 381 91 H-I 297 71 444 106 HO-H 498 119 331 79 НО -ОН 213 51 272 65 Methyl bonds (CH3)3C-I (CH3)3C-OH 209 50 381 91 CH3-H CH3-F 435 104 456 109 Other C-H bonds CH3-CI 351 84 PHCH,–H (benzylic) 356 85 CH3-Br 293 70 CH,— СНCH, — н (allylic) 364 87 CH;-I 234 56 CH,=CH-H (vinyl) 464 111 CH — ОН 381 91 Ph-H (aromatic) 473 113 Bonds to primary carbons НС-С- Н (асetylenic) 523 125 CH;CH2-H 410 98 CH;CH2-F 448 107 C-C bonds CH3CH2-CI 339 81 CH3-CH3 368 88 CH3CH2-Br 285 68 CH;CH,-CH; 356 85 CH;CH2-I CH;CH2-OH 222 53 CH,CH,-CH,CH3 343 82 381 91 (CH3)2CH-CH3 351 84 CH;CH,CH2-H CH;CH,CH2-F 410 98 (CH3);C-CH3 339 81 448 107 CH;CH,CH,-CI CH;CH,CH2- Br CH;CH,CH2-I 339 81 285 68 222 53 CH;CH,CH2-OH 381 91
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
In the chlorination of isobutene there are two possible first propagation steps They are ...View the full answer

Answered By

Amit Rathod
Hello, my name is Amit Rathod, I have one year of teaching experience, I solve questions in Computer Science & Mathematics Subject. I like to solve most of the difficult questions so that my subject knowledge can be increased and students can also get help.
Education: Bachelor of Engineering / Information Technology
University: Gujarat Technological University
I will complete my engineering studies in 2022.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Using bond-dissociation enthalpies from Table 4-2 (page 143), calculate the heat of reaction for each step in the free-radical bromination of methane. (b) Calculate the overall heat of reaction....
-
Carbon-carbon bond dissociation energies have been measured for alkanes. Without referring to Table 4.3, identify the alkane in each of the following pairs that has the lower carbon-carbon bond...
-
Use average bond enthalpies (Table 8.4) to estimate for the atomization of benzene, C6H6: C6H6 (g) 6C (g) + 6 H (g) Compare the value to that obtained by using Hof data given in Appendix C and...
-
Each of the following passages may be plausibly criticized by some who conclude that it contains a fallacy, but each may be defended by some who deny that the argument is fallacious. Discuss the...
-
(a) Would you need NaOH or HCl to bring the pH of 0.050 0 M HEPES (Table 8-2) to 7.45? (b) Describe how to prepare 0.250 L of 0.050 0 M HEPES, pH 7.45.
-
What are the best working hours for the Net Generation?
-
Revenues of popular movies. The Internet Movie Database (www.imdb.com) monitors the gross revenues of all major motion pictures. The table on p. 791 gives both the domestic (United States and Canada)...
-
Nathan sells gourmet hot dogs. His customers have identical inverse demands, given by P = 5 .25Q. Nathan can produce hot dogs at a constant marginal and average cost of $1. a. If Nathan operates as...
-
Harbor Co. began constructing a building for its own use in January of the current year. During the current year, Harbor incurred interest of $45,000 on specific construction debt, and $30,000 on...
-
Failure to follow accounting principles causes immense confusion, which in turn creates a number of problems for the organization. Those with vast experience in bookkeeping, however, are able to...
-
Write structures for a homologous series of alcohols (R-OH) having from one to six carbons.
-
Predict the ratios of products that result from chlorination of isopentane (2-methylbutane).
-
Which of the destinations and attractions identified in the last section are primary destinations? Which are secondary?
-
1. The interest rate charged on a loan of $85,000 is 7.75% compounded annually. If the loan is to be paid off over seven years, calculate the size of the annual payments. 2. A $10,000 debt is repaid...
-
Referring to the NISSAN Navara 5L SE M/T, which is using Nissan YD25 engine, provide your analysis to the following questions: A. What will be the maximum power produced by this vehicle at the...
-
Convert each pair of rectangular coordinates to polar coordinates where r> 0 and 0 <2.
-
Please type your answers and submit them on Blackboard by the due time. AAA corp. had the following PP&E values on Dec. 31, 2018. Cost $ 100 Accumulated Depreciation $ 20 Undiscounted Future Cash...
-
Compute the standard deviation"sigma symbol"for ages of British nurses in 1851. Assume that the table below shows the age distribution of nurses in Great Britain in 1851. Round your answer to nearest...
-
The rotation of the gear is controlled by the horizontal motion of end A of the rack AB. If the piston rod has a constant velocity x = 300 mm/s during a short interval of motion, determine the...
-
The Ferris wheel in the figure has a radius of 68 feet. The clearance between the wheel and the ground is 14 feet. The rectangular coordinate system shown has its origin on the ground directly below...
-
The drug mechktrethamine is used in antitumor therapy. It is one of a family of compounds called nitrogen mustctrds, which also includes the antitumor drugs cyclophosphamide and chlorambucil. (a)...
-
In each of the following pairs , one. of the glycols is virtually inert to periodate oxidation. Which glycol is inefi? Explain why. CH3 CH, or
-
Provide a curved-arrow mechanism for each of the reactions in Fig. Pl1.73 that accounts for the stereochemical results. Show the structure of the unstable intermediate in each case and explain why it...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


