The drug mechktrethamine is used in antitumor therapy. It is one of a family of compounds called
Question:
The drug mechktrethamine is used in antitumor therapy.
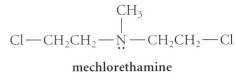
It is one of a family of compounds called nitrogen mustctrds, which also includes the antitumor drugs cyclophosphamide and chlorambucil.
(a) Mechlorethamine undergoes a nucleophilic substitution reaction with water that is several thousand times faster than the nucleophilic substitution reaction of 1,5-dichloropentane. Give the product of the mechlorethamine reaction and the mechanism for its formation.
(b) It is theorized that the antitumor effects of mechlorethamine are due to its reaction with certain nucleophiles in the body. What procluct would you expect from the reaction of mechlorethamine and a general amine R,N:?
Transcribed Image Text:
CH mechlorethamine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a The rate acceleration suggests a mechanism involving neigh...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
For each of the following reactions, predict the major product and propose a mechanism for its formation. (a) (b) (c) :? 1) LDA 2) CH3I 1) NaH 2) -CH,Br
-
For each of the following transformations, predict the major product, and draw a mechanism for its formation: (a) (b) 1) xs NANH,/NH3 Br 2) H,0 Br CI 1) xs NANH,/NH3 2) H,0
-
Assign configuration to the following substance, and draw the structure of the product that would result on nucleophilic substitution reaction with HS ? (reddish brown = Br):
-
Explain incremental cash flow, externality? Why is timing of cash flows important? What is scenario analysis? Would a project's NPV for a firm be higher or lower if the firm used accelerated rather...
-
Eastern Digital Corp. has a convertible bond outstanding with a coupon rate of 9 percent and a maturity date of 20 years. It is rated Aa, and competitive, nonconvertible bonds of the same risk class...
-
Identify and explain at least four strategies of managerial influence. Give examples of how each strategy may or may not work when one is using influence in organizations (a) downward and (b) upward.
-
Make up a classification scheme that is inherently flawed, and would lead to misclassification, as we find in Table 2.2. For example, classes of items bought in a grocery store.
-
A continuous distillation operation with a reflux ratio (L/D) of 3.5 yields a distillate containing 97 wt% B (benzene) and bottoms containing 98 wt% T (toluene). Due to weld failures, the 10 plates...
-
Pronghorn Inc., a greeting card company, had the following statements prepared as of December 31, 2020. PRONGHORN INC. COMPARATIVE BALANCE SHEET AS OF DECEMBER 31, 2020 AND 2019 12/31/20 12/31/19...
-
Dry Quick (DQ) is a medium-sized, private manufacturing company located near Timmins, Ontario. DQ has a June 30 year-end. Your firm, Poivre & Sel (P&S), has recently been appointed as auditors forDQ....
-
Explain why the deydration of primary alcohols can only be used for preparing symmetri, cal ethers. What would happen if a mixture of two different alcohols were used as the starting material in this...
-
In each of the following pairs , one. of the glycols is virtually inert to periodate oxidation. Which glycol is inefi? Explain why. CH3 CH, or
-
Express each complex number in its polar form. \(-\frac{2}{3}+j\)
-
An employer has calculated the following amounts for an employee during the last week of June 2021. Gross Wages $1,800.00 Income Taxes $414.00 Canada Pension Plan $94.00 Employment Insurance $28.00...
-
Section Two: CASE ANALYSIS (Marks: 5) Please read the following case and answer the two questions given at the end of the case. Zara's Competitive Advantage Fashion houses such as Armani and Gucci...
-
The activity of carbon in liquid iron-carbon alloys is determined by equilibration of CO/CO2 gas mixtures with the melt. Experimentally at PT = 1 atm, and 1560C (1833 K) the equilibrated gas...
-
Apply knowledge of concepts and theories covered in the course to leader - the leader can either be themselves if they lead a team, someone real and personally known to them (such as a boss or leader...
-
A resistor in a dc circuit R = 1.2 2. The power dissipated P is a second-degree function of the voltage V. Graph P versus V from V = 0.0 V to V = 3.0 V.
-
Why were some leases referred to as off-balance-sheet financing prior to 2019?
-
Assume that your audit team has established the following parameters for the examination of ELM's sales transactions: LO G-3 Risk of incorrect acceptance...
-
Allene (1, 2-propadiene), H2C = C = CH2, has two adjacent double bonds. What kind of hybridization must the central carbon have? Sketch the bonding orbitals in allene. What shape do you predict for...
-
The heat of hydrogenation for allene (Problem 6.37) to yield propane is 295kJ/mol, and the heat of hydrogenation for a typical monosubstituted alkene such as propene is 126kJ/mol. Is allene more...
-
Predict the major product in each of the following reactions: CH (a) H20 CHCH-CH3H2CH H2SO4 (Addition of H20 occurs.) (b) CH-CH CH (c) HBr (d) 2 HCI %3 CHCH2CH2CH2CH%3DCH2
-
help!!! Use the above information to calculate ending inventory using FIFO for a company that uses a perpetua/inventory system
-
Rocky Mountain Chocolate Factory (RMCF) founder and president Frank Crail employs 220 people in 361 outlets in the United States, Canada, United Arab Emirates, Japan, South Korea and Saudi Arabia. If...
-
The market price of a semi-annual pay bond is $979.86. It has 21.00 years to maturity and a yield to maturity of 7.34%. What is the coupon rate? Submit Answer format: Percentage Round to: 0 decimal...

Study smarter with the SolutionInn App


