When an unprotected -amino acid is treated with dicyclohexylcarbodiimide (DCC), a 2,5-diketopiperazine results. Explain. HN HR O
Question:
When an unprotected -amino acid is treated with dicyclohexylcarbodiimide (DCC), a 2,5-diketopiperazine results. Explain. 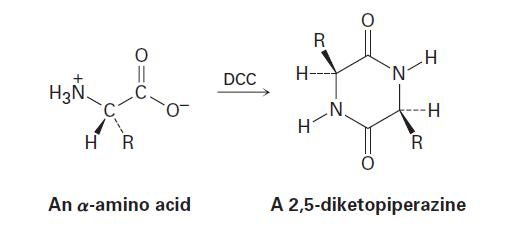
Transcribed Image Text:
H₂N HR O An a-amino acid DCC R HJ H- -N H- N -H R A 2,5-diketopiperazine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
When an unprotected amino acid is treated with dicyclohexylcarbodiimide DCC a 25diketopiperazine DKP ...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When N - acetyl- L -aspartic acid is treated with acetic anhydride, an optically active compound A, C6H7NO4, is formed" Tleaffient of A with the amino acid L -alanine yields two separable, isomeric...
-
The phenomenon of configuration inversion in a chemical reaction was discovered in 1896 by Paul Walden (Section 6.6). Walden's proof of configuration inversion was based on the following cycle: (a)...
-
When an extract of parsley seed is saponified and acidified, one of the fatty acids isolated is petroselenic acid, formula C18H34O2. Hydrogenation of petroselenic acid gives pure stearic acid. When...
-
At one time the Thames River in England supported an abundant community of fish. Pollution then destroyed all the fish in a 40-mile stretch near its mouth for a 45-year period beginning in 1915....
-
You plan to retire at age 40 after a highly successful but short career. You would like to accumulate enough money by age 40 to withdraw $225,000 per year for 40 years. You plan to pay into your...
-
Use a double integral to find the area of the shaded region. R|N I 2 r = 1 + cos 0 1 0
-
Find the equation of the regression line for the data. Draw the regression line on the scatter plot.
-
At December 31, MediSharp Precision Instruments owes $50,000 on accounts payable, salary payable of $16,000, and income tax payable of $8,000. MediSharp also has $280,000 of bonds payable that were...
-
Choose an Excel function/skill with Finance applications such as VBA and demonstrate and explain the topic/skill as if you are training co-workers . The content can be a function/skill you learned at...
-
The mosquito attractant oct-1-en-3-ol can be prepared by reduction of oct-1-en-3-one. How can UV spectroscopy be used to show when the reaction is complete?
-
The amino acid analysis data in Figure 15.2 indicate that proline is not easily detected by reaction with ninhydrin. Suggest a reason. Absorbance Asp 0.0 10.0 Thr Ser Ile Leu Tyr Glu Met Gly Val Ala...
-
Kelley Company reports $960,000 of net income and declares $120,000 of cash dividends on its preferred stock for the year. At year-end, the company had 400,000 weighted-average shares of common...
-
The following information was obtained from the records of Shae Inc.: Merchandise inventory $ 88,000 Notes payable (long-term) 100,000 Net sales 300,000 Buildings and equipment 168,000 Selling,...
-
Absent Clothing Company Savita Kapur, CEO, founded Absent Clothing Company (ACC) in 2005. ACC sells practical athletic wear to service the yoga and pilates market. Savita originally created ACC with...
-
Find the indicated area under the curve of the standard normal distribution; then convert it to a percentage and fill in the blank. About % of the area is between z = - 3.5 and z = 3.5 (or...
-
EM 605 Spring 2021 Midterm Exam 3/17/2021 The linear programming problem whose output follows is used to determine how many bottles of Hell-bound red nail polish (x1), Blood red nail polish (x2),...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Prove Euler's theorem that, if f(L, K) is homogeneous of degree ( (see Exercise 5.7), then L((f/(L) + K((f/(K) = (((L, K). Given this result, what can you conclude if a production function has...
-
Pearson Education, a publisher of college textbooks, would like to know if students prefer traditional textbooks or digital textbooks. A random sample of students was asked their preference and the...
-
Which of the following pure compounds will exhibit hydrogen bonding? a) CH 3 CH 2 OH b) CH 2 O c) C 2 H 4 d) C 2 H 2 e) CH 3 OCH 3 f) CH 3 NH 2 g) C 3 H 8 h) NH 3
-
For each case below, identify the most likely value for x: a) BH x b) CH x c) NH x d) CH 2 Cl x
-
Identify the hybridization state and geometry of each carbon atom in the following compounds: a. b. c. -OEJ- -CEC-C . .C. H. 1 H' .
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


