When it is strongly heated, ethyl diazoacetate decomposes to give nitrogen gas and a carbene. Draw a
Question:
When it is strongly heated, ethyl diazoacetate decomposes to give nitrogen gas and a carbene. Draw a Lewis structure of the carbene.
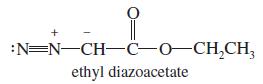
Transcribed Image Text:
:N=N-CH-C-O-CH,CH, ethyl diazoacetate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When dinitrogen pentoxide, N2O5, a white solid, is heated, it decomposes to nitrogen dioxide and oxygen. If a sample of N2O5 produces 1.315 g O2, how many grams of NO2 are formed? 2N205(s) 4NO2(g) +...
-
The molecule benzyne (C6H4) is a very reactive species. It resembles benzene in that it has a sixmembered ring of carbon atoms. Draw a Lewis structure of the molecule and account for the molecule's...
-
Ethylene oxide, C2H4O, decomposes when heated to give methane and carbon monoxide. C2H4O(g) CH4(g) + CO(g) The following kinetic data were observed for the reaction at 688 K: Find the rate law and...
-
In Exercises determine whether the statement is true or false. If it is false, explain why or give an example that shows it is false. The curve represented by the parametric equations x = t and y =...
-
Calculate the pH of 5.0 10-8 M HClO4. What fraction of the total H+ in this solution is derived from dissociation of water?
-
Which formatting choice for Excels Conditional Formatting tool can be used to create a heat map? a) icon sets b) changing the fill color of cells c) changing font type d) data bars e) color scales L01
-
Calculate the IRR for the following project. An outflow of $14,000 in year 0 was followed by an inflow of 0 for 2 years with $16,000 returned in the third year. How does this change if the cash flows...
-
Monica Sexton filed a petition for Chapter 13 reorganization. One of her creditors was Friedmans Jewelers. Her petition misclassified Friedmans claim as $ 800 of unsecured debt. Within days,...
-
A chain of appliance stores, APP Corporation purchases inventory with a net price of $200000 each day. The company purchases the inventory under the credit terms of 1/15, net 35. APP always takes the...
-
Elysian Fields, Inc., uses a maximum payback period of 6 years and currently must choose between two mutually exclusive projects. Project Hydrogen requires an initial outlay of $25,000; project...
-
Write an equation for the reaction of vitamin E with an oxidizing radical (RO) to give ROH and a less reactive free radical.
-
A chiral sample gives a rotation that is close to 180. How can one tell whether this rotation is +180 or -180?
-
A London-based investor wants to estimate roll-down return attributable to a fixed-rate, option-free corporate bond versus UK gilts over the next six months assuming a static, upward-sloping...
-
Required: Prepare the supporting schedules for your portfolio for presentation to Mandla the supervisor and senior administrator. The schedules for the portfolio need to cover the following: Part A...
-
Write a program that will predict the size of a population of organisms. The program should ask for the starting number of organisms, their average daily population increase (as a percentage), and...
-
Management is keen to reduce inventory levels for materials as well and closing inventories are to be much lower. Expected levels are shown below: Material M1 Material M2 Material M3 2,200 kg 1,300...
-
How do you calculate incremental cost for the following: Complying with the Clean Air Act Amendments will be costly. There are three main options for complying with the Clean Air Act: analyze the...
-
How do I journalize this transaction? Mountain Swirl Ice Cream purchased and took delivery of one ice cream machine for $7,500. Record the sale and the cost of the sale. Markup is 150% of cost....
-
The elements of the mechanism for deployment of a spacecraft magnetometer boom are shown. Determine the angular velocity of the boom when the driving link OB crosses the y-axis with an angular...
-
Evaluate each logarithm to four decimal places. log 0.257
-
(a) You have found in the laboratory two liquids, C and D, in unlabeled bottles. You suspect that one is deuterated chloroform (CDC13) and the other is ordinary chloroform (CHC13). Unfortunately, the...
-
Rationalize the indicated fragments in the EI mass spectrum of each of the following molecules by proposing a structure of the fragment and a mechanism by which it is produced. (a)...
-
A chemist, Ilov Boronin, carried out a reaction of frans-2-pentene with BH3 in THF followed by treatment with H2O2/-OH. Two products were separated and isolated. Desperate to know their structures,...
-
Los datos de la columna C tienen caracteres no imprimibles antes y despus de los datos contenidos en cada celda. En la celda G2, ingrese una frmula para eliminar cualquier carcter no imprimible de la...
-
Explain impacts of changing FIFO method to weighted average method in inventory cost valuations? Explain impacts of changing Weighted average method to FIFO method in inventory cost valuations?...
-
A perpetuity makes payments starting five years from today. The first payment is 1000 and each payment thereafter increases by k (in %) (which is less than the effective annual interest rate) per...

Study smarter with the SolutionInn App



