Where would you expect to find the 1 H NMR signal of (CH 3 ) 2 Mg
Question:
Where would you expect to find the 1H NMR signal of (CH3)2Mg relative to the TMS signal? (See Table 12.3.)
Table 12.3
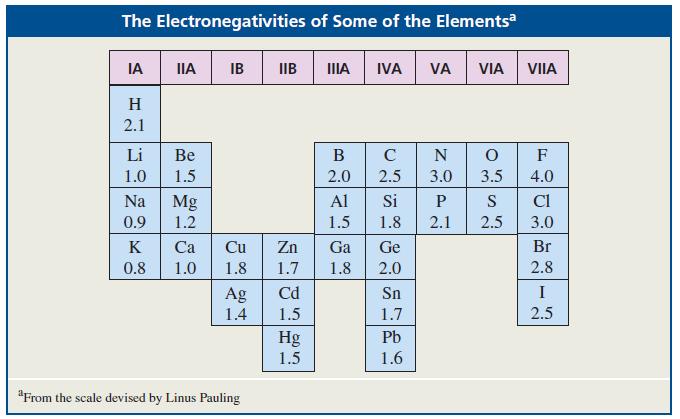
Transcribed Image Text:
The Electronegativities of Some of the Elementsa IA IIA IB IIB А IVA VA VIA VIIA H 2.1 Li Ве В C N F 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Na Mg Al Si P S CI 0.9 1.2 1.5 1.8 2.1 2.5 3.0 K Са Cu Zn Ga Ge Br 0.8 1.0 1.8 1.7 1.8 2.0 2.8 Cd I Ag 1.4 Sn 1.5 1.7 2.5 Pb Hg 1.5 1.6 "From the scale devised by Linus Pauling
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
Because Mg is less electronegative than Si the methy...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In what chemical shift ranges would you expect to find the proton NMR signals of ethyl acetate (CH3CO2CH2CH3)?
-
How many signals would you expect to find in the 1H NMR spectrum of each of the following compounds? (a) 1-Butanol (b) Butane (c) 1,4-Dibromobutane
-
(a) How many signals would you expect to find in the 1H NMR spectrum of caffeine? (b) What characteristic peaks would you expect to find in the IR spectrum of caffeine? CH3 CH3 Caffeine
-
Refer to the RMO CSMS Order Fulfillment subsystem shown in Figure. Draw a use case diagram that shows all actors and all use cases. Use a drawing tool such as Microsoft Visio if it is available.
-
The exitance (power per unit area per unit wavelength) from a blackbody (Box 19-1) is given by the Planck distribution: where _ is wavelength, T is temperature (K), h is Planck's constant, c is the...
-
What is a high-performance work system? What are its elements? Which of these elements involve human resource management? (LO 9-1)
-
Refer again to the drug reaction regression. Predict the reaction time for the LO9 next performance of the experiment for a subject with a drug concentration of 4%. Use a 95% prediction interval.
-
The Village of Delmar is preparing its government- wide statement of activities for the year ended December 31, 2013. Analysis of the data accumulated thus far shows the following expenses for each...
-
On January 14 2016, Nokia and Alcatel-Lucent celebrated their first day of combined operations. A couple of years earlier, Nokia and Microsoft formed a partnership, which did not work well. Why did...
-
Your tax clients, Jack and Diane a married couple filing a joint return, fell in love with a new construction house for sale in their small Illinois hometown. The builder offers two options on the...
-
How could you distinguish the 1 H NMR spectra of the following compounds? a. CH 3 OCH 2 OCH 3 b. CH 3 OCH 3 c. CH3 CH3OCH,CCH2OCH3 H3
-
a. Calculate the ratios of the different kinds of protons in a compound with an integral ratio of 6 : 4 : 18.4 (going from left to right across the spectrum). b. Determine the structure of a compound...
-
Consider the round-trip transactions recorded by Time Warner described in the chapter. a. Describe how the company committed fraud. b. What type of fraud did it commit? c. What was the auditors...
-
21. How a degradation process is modeled? 22.Give the homogenity property in Linear Operator 23. Give the relation for degradation model for continuous function 24.which is called the superposition...
-
28. Define Gray-level interpolation 29. What is meant by Noise probability density function? 30. Why the restoration is called as unconstrained restoration? 31. Which is the most frequent method to...
-
34. Give the relation for guassian noise 35. Give the relation for rayleigh noise 36. Give the relation for Gamma noise 37. Give the relation for Exponential noise 38. Give the relation for Uniform...
-
41. What is pseudo inverse filter? 42. What is meant by least mean square filter? 43. Give the difference between Enhancement and Restoration PART-B 1. Discuss different mean filters
-
1.Discuss different mean filters 2. Draw the degradation model and explain. 3.Write short notes on Median Filters
-
Calculate the iterated integral. 2 2 + 2xe") dx dy
-
(a) Prove that form an orthonormal basis for R3 for the usual dot product. (b) Find the coordinates of v = (1, 1, 1)T relative to this basis. (c) Verify formula (5.5) in this particular case. 48-65...
-
Give Lewis structures corresponding to the following line-angle structures. Give the molecular formula for each structure. (a) (b) (c) (d) (e) (f) (g) (h) OH CHO
-
Repeat Problem 1-9, this time drawing line-angle structures for compounds (a) through (h). In problem 1-9 Draw complete Lewis structures for the following condensed structural formulas. (a)...
-
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula. C1 (a) (b) (c) (d) 40.0% 32.0%...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


