Which of the following compounds have delocalized electrons? a. b. c. d. e. CH 2 =CHCH 2
Question:
Which of the following compounds have delocalized electrons?
a.
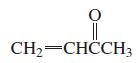
b.
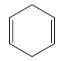
c.
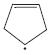
d.
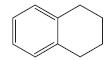
e. CH2=CHCH2CH=CH2
f.
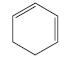
g.
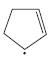
h. CH3CH2NHCH2CH=CHCH3
i. CH3CH2NHCH=CHCH3
j.
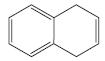
k.
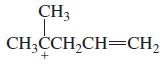
Transcribed Image Text:
CH2-CHCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
For delocalisation of electrons or Resonance compound should contain conjugation ...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. Which of the following compounds have delocalized electrons? 1. 2. 3. 4. CH2==CHCH2CH==CH2 5. 6. 7. CH3CH2NHCH2CH==CH2 b. Draw the contributing resonance structures for these compounds. NH2...
-
Which of the following compounds are aromatic? a. b. c. Cycloheptatrienyl cation d. e. f. g. Cyclononatetraenyl anion h. CH2=CHCH=CHCH=CH2
-
Which of the following compounds have a dipole moment of zero? CI H CI H C-C C-C C-C C-C CICI H C H CI Cl
-
Can you provide an example of when you led a previous organization through a major operational change? Did you encounter any obstacles and how did you motivate and guide your team through these...
-
An electron volt (eV) is the energy change of an electron moved through a potential difference of 1 volt: eV (1.602 Ã 10-19C)(1 V) 1.602 Ã 10-19 J per electron 96.49 KJ per mole of...
-
Describe the profile of a management consultant. LO.1
-
Effect of massage on boxers. Refer to the British Journal of Sports Medicine (Apr. 2000) study of the effect of massage on boxing performance, presented in Exercise 10.82 LO9 (p. 580). Two other...
-
CVP analysis (CMA, adapted) Galaxy Disks projected operating income for 2008 is $200,000, based on a sales volume of 200,000 units. Galaxy sells disks for $16 each. Variable costs consist of the $10...
-
Leah, Inc., is proposing a rights offering. Presently there are 400,000 shares outstanding at $44 each. There will be 100,000 new shares offered at $35 each. What is the new market value of the...
-
Consider the competitive market for steel. Assume that, regardless of how many firms are in the industry, every firm in the industry is identical and faces the marginal cost (MC), average total cost...
-
Which of the following would you predict to be the stronger acid? -- or N- C-OH
-
Draw resonance contributors for the following ions: a. b. c. d.
-
Identify two liability categories on the classified balance sheet, and give examples of each category.
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Estimate the hydrogen consumption required to completely remove the sulfur from a hydrotreater feedstock and to reduce the nitrogen content of the product to 15 ppm by weight. The 48.5 API naphtha...
-
2. Consider the following kinds of information, and suggest the most appropriate data type to store or represent each: Information Suggested Data Type String A person's name A person's age in years A...
-
Steam at 32 MPa, 520C enters the first stage of a supercritical reheat cycle including three turbine stages. Steam exiting the first-stage turbine at pressure p is reheated at constant pressure to...
-
Investigate the shape of the surface with parametric equations x = sin u, y = sin v, z = sin(u + v). Start by graphing the surface from several points of view. Explain the appearance of the graphs by...
-
You deposit $10,000 in a savings account that earns 7.5% simple interest per year. What is the minimum number of years you must wait to double your balance? Suppose instead that you deposit the...
-
After the coupling, he deprotects his resin-bound peptide with 20% piperidine in DMF, and then completes the synthesis in the usual way by coupling Fmoc-Gly, deprotecting the peptide, and removing it...
-
Consider the following solid-phase peptide synthesis: (a) Give the structure of each compound A-P. (b) Explain the reason for the Boc group on the side chain of the Lys group in the reaction B C (c)...
-
Consider the following solid-phase peptide synthesis: (a) Give the structure of each compound A-P. (b) Explain the reason for the Boc group on the side chain of the Lys group in the reaction B C (c)...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


