Which of the following pairs are ketoenol tautomers? a. b. c. d. e. CH;CH,CH=CHCH,OH and CH;CH,CH,CH,CH
Question:
Which of the following pairs are keto–enol tautomers?
a.
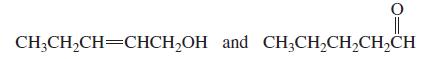
b.
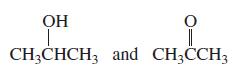
c.
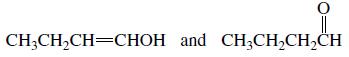
d.

e.
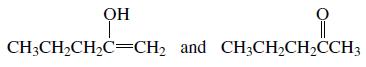
Transcribed Image Text:
CH;CH,CH=CHCH,OH and CH;CH,CH,CH,CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Keto enol tautomerism It is a chemical equilibricom bet keto ...View the full answer

Answered By

Shantanu Jana
I have completed my schooling from contai model institution....after that I completed my graduation from vidyasagar university on chemistry....after that now I am studying in jadavpur university on chemistry.....I am going through teaching for many years since my graduation....so I hope I will be able to taught them.... I just won't be there teacher there I will also guide them to be a better human besides being a brilliant student....I hope they will cooperate with me....
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify which of the following compounds represent a pair of keto-enol tautomers: (a) (b) (c) (d) -OH
-
In each of the following pairs of compounds, choose the one that has the greater enol content, and write the structure of its enol form: (a) (b) (c) (d) (e) (f) (CH)3CCH or (CH2CHCH CH CC H or...
-
Draw the enol tautomers for each of the following compounds. For those compounds that have more than one enol tautomer, indicate which is more stable. a. b. c. d. e. f. CH3CH2CCH,CH3 CCH3 CH CH...
-
Which market segments are the most favorable for Hyundai card? Does the company do an effective job of targeting and reaching those segments? If so, How? If not, why not?
-
The 20-m-radius laser ablation pit in Figure 20-28 was created by a laser pulse with a duration of 10 ns and an energy of 2.4 mJ. Express the laser power density in units of W/cm2. Recall that 1 W 1...
-
When is it most likely for a hospitality business to hire a consultant? List three situations. LO.1
-
Ideal height of your mate. Refer to the Chance (Summer 2008) study of the height of the ideal mate, Exercise 11.33 (p. 629). You used the data to fit the simple linear regression LO9 model E1y2 = b0...
-
What is the present value cost of owning the equipment? set up a time line which shows the net cash flows over the period t = 0 to t = 4, and then find the PV of these net cash flows, or the pv cost...
-
a. Prepare a multiple-step income statement for the fiscal year ended March 31, 20Y9. b. What is a major advantage of the multiple-step income statement over the single-step income statement
-
Encoding and decoding schemes are used in a wide variety of applications, such as in the streaming of music and videos, data communications, storage systems (e.g., on CDs, DVDs, RAID arrays), among...
-
A chemist wants to synthesize 4-decyne but cannot find any 1-pentyne, the starting material used in the synthesis just described. How else can 4-decyne be synthesized?
-
Which of the following would you predict to be the stronger acid? -- or N- C-OH
-
Weighted Average Repeat requirement | of the preceding problem consistent with the weighted-average method for treating product costs and consistent with the companys treatment of spoilage and lost...
-
Salmone Company reported the following purchases and sales of its only product. Salmone uses a perpetual inventory system. Determine the cost assigned to the ending inventory using FIFO. 1 Date...
-
A company may go through organizational change at various stages in its life cycle for a variety of reasons. Reasons can include a change in ownership as well as a change in the competitive...
-
6 (a) Below is a diagram of a rotating disc viscometer (FIGURE 4). Explain its operations and limitations as to use. If, in a similar works situation, it is necessary to make measurements on a...
-
As part of your role in the Business Analytics and Data Analytics team, you have been asked to forecast Food Retailing as part of a wider report being commissioned by the above collaboration - on...
-
You are three students who have together bought a business that makes snow. The customers consist of both large public enterprises and private individuals. The business is run all year round, but the...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. If f has continuous partial derivatives on R...
-
Suppose the concentration of glucose inside a cell is 0.1 mm and the cell is suspended in a glucose solution of 0.01 mm. a. What would be the free energy change involved in transporting 10-o mole of...
-
A mixture of N-acetyl-Leu-Gly, Lys-Gly-Arg, and Lys-Gly-Leu is applied to a sulfonated polystyrene cation-exchange column at a buffer pH of 6.0. Predict the order in which these three peptides will...
-
Indicole which of the method in this section could be used to prepare each of the following amino acids. For each method that can be used, give an equation. For each case in which a method would not...
-
Give the major product expected when (a) Leucine is treated with p-toluenesulfonyl chloride (tosyl chloride). (b) Alanine is heated in methanol solvent with HCI catalyst.
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


