(a) Aliphatic allylic vinylic ethers undergo the Claisen rearrangement. Complete the following reaction: (b) What starting material...
Question:
(a) Aliphatic allylic vinylic ethers undergo the Claisen rearrangement. Complete the following reaction:
![]()
(b) What starting material would give the following compound in an aliphatic Claisen rearrangement?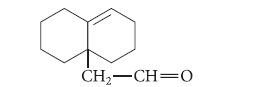
Transcribed Image Text:
(CH;),C=CH–CH, O–CH=CH, heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
a Deduce the product by using the curvedarrow ...View the full answer

Answered By

Gaurav Soni
Myself a student of Physics currently pursuing my PG. I helped many students due to my understanding specifically among students and also in my subject i. e. Physics. Helping them in various boards and in various entrance examination gives me a satisfaction of gaining knowledge. I have taught physics to students for many engineering and medical entrance examination and also for various boards in india. Till now have a great results from my students and that's why having a good impact on them. I have a good knowledge in conceptual and theoretical physics. Essential University Physics is one of my favorite field of teaching. I have a experience of almost 4 years in teaching.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What starting material would give the following compound in an aliphatic Claisen rearrangement? CH,-CH-C
-
The mechanism of the Claisen rearrangement of other allylic ethers of phenol is analogous to that of allyl phenyl ether. What is the product of the Claisen rearrangement of C6H5OCH2CH==CHCH3?
-
The mechanism of the Claisen rearrangement of other allylic ethers of phenol is analogous to that of allyl phenyl ether. What is the product of the Claisen rearrangement of C6H5OCH2CH=CHCH3?
-
You have observed the following returns over time: Assume that the risk-free rate is 6% and the market risk premium is 5%. a. What are the betas of Stocks X and Y? b. What are the required rates of...
-
A recent annual report for FedEx includes the following information: For financial reporting purposes, we record depreciation and amortization of property and equipment on a straight-line basis over...
-
Find the interest rates earned on each of the following: a. You borrow $720 and promise to pay back $792 at the end of 1 year. b. You lend $720 and the borrower promises to pay you $792 at the end of...
-
From the following budgeted and actual figures, calculate the variances on sales margin basis: Budget Sales2,000 units at Rs 15 each Rs 30,000 Cost of sales at Rs 12 each Rs 24,000 Profit Rs 6,000...
-
Simco Blenders makes different kinds of electrical blenders, mixers, and grinders for various kitchen needs. These products are powered by small electric motors. Presently, Simco buys these motors...
-
Required information Use the following information for the Exercises below. ( Algo ) [ The following information applies to the questions displayed below. ] BMX Company has one employee. FICA Social...
-
Show how the transition state for a [3,3] sigmatropic reaction can be analyzed as the interaction of two allylic radicals, and that the same stereochemical outcome is predicted.
-
(a) What allowed and reasonable sigmatropic reaction(s) can account for the following transformation? (b) What product(s) are expected from a similar reaction of 2,3-dimethyl-1,3- cyclopentadiene?...
-
How many acres are there in a rectangular property measuring 363 ft by 180 ft?
-
4. What is the time complexity of the following procedure for in/2 to n do j 2 end for while (j
-
If the concentration of a constituent in the influent to the equalization basin is constant over the 24 h period, will the load of the constituent from the basin be constant? If the concentration of...
-
A three-phase transmission line of a 60 Hz circuit has a length of 370 km (230 miles). the conductors are of the 795,000cm (54/7) type with horizontal spacing of 25 feet between them. The load on the...
-
Simulate rolling a dice using Math.random() . Your roll function should allow the caller to specify any number of sides, but default to 6 if no side count is given: roll() assumes a 6 sided dice,...
-
Drama Read the excerpt from a play. Then, answer the question(s). (1) (2) Belle: Having trouble deciding what will make you look like both a power to be reckoned with and a fetching young lady while...
-
Walthman Industries Inc. employs seven salespersons to sell and distribute its product throughout the state. Data taken from reports received from the salespersons during the year ended December 31...
-
It is possible to investigate the thermo chemical properties of hydrocarbons with molecular modeling methods. (a) Use electronic structure software to predict cHo values for the alkanes methane...
-
For which of the following ions does the formal charge give a fairly accurate picture of where the charge really is? Explain in each case. (a) NH4 (b) H3O: (C) NH2 (d) CH3
-
Is there an unbranched alkane containing 23 hydrogen atoms? If so, give its structural formula; if not, explain why not.
-
In the structure of 4- isopropy 1-2,4,5-trimethylheptane (Problem 2.9) (a) Identify the primary, secondary, tertiary, and quaternary carbons. (b) Identify the primary, secondary, and tertiary...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


