(a) Explain why compound A, when treated with one equivalent of NaOEt, followed by acidification, is completely...
Question:
(a) Explain why compound A, when treated with one equivalent of NaOEt, followed by acidification, is completely converted into compound B.
(b) Write a curved-arrow mechanism for this conversion.
(c) Give the structure of the only product formed when diethyl a-methyladipate (compound C) reacts in the Dieckmann condensation. Explain your reasoning.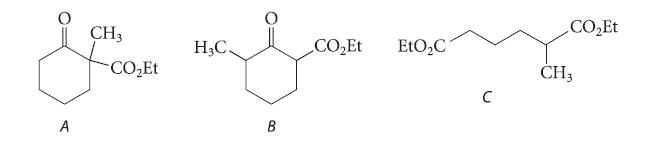
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





