(a) In the products of Eq. 15.14, the observed stereochemistry at the ring fusion is not specified....
Question:
(a) In the products of Eq. 15.14, the observed stereochemistry at the ring fusion is not specified. Show this stereo chemistry, assuming that the Diels–Alder reaction gives the endo product.
(b) Sketch diagrams like the one in Fig. 15.10 (without the orbitals) that shows the approach of the diene and dienophile leading to both endo and exo products in part (a). Pay careful attention to the relative positions of substituent groups.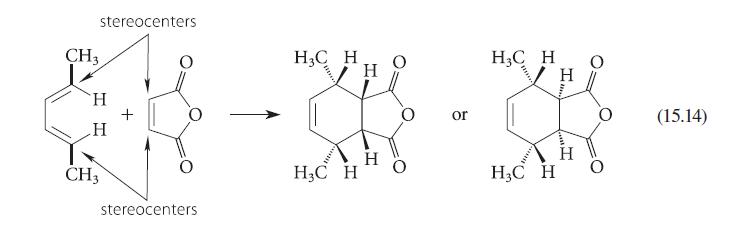
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





