(a) The pK a of 2-nitropropane is 10. Give the structure of its conjugate base, and suggest...
Question:
(a) The pKa of 2-nitropropane is 10. Give the structure of its conjugate base, and suggest reason(s) why 2-nitropropane has a particularly acidic C—H bond.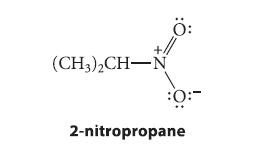
(b) When the conjugate base of 2-nitropropane is protonated, an isomer of 2-nitropropane is formed, which, on standing, is slowly converted into 2-nitropropane itself. Give the structure of this isomer.
(c) What product forms when 2-nitropropane reacts with ethyl acrylate (H2C=CH—CO2Et) in the presence of NaOEt in EtOH?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





