(a) Use the relative bond lengths of the CC and CO bonds to predict which of the...
Question:
(a) Use the relative bond lengths of the C—C and C—O bonds to predict which of the following two equilibria lies farther to the right. (That is, predict which of the two compounds contains more of the conformation with the axial methyl group.)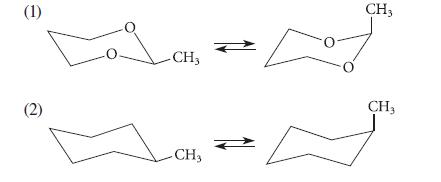
(b) Which one of the following compounds contains the greater amount of gauche conformation for internal rotation about the bond shown? Explain.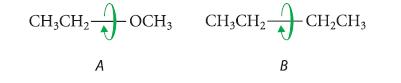
Transcribed Image Text:
(1) (2) -CH3 - CH3 ← CH3 CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Equilibrium 2 contains more of the conformation with the methyl group in an axial position than eq...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
a. Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (CO23-) b. What would you expect the charge to be on each oxygen atom?
-
Kenny operates a store, where he sells feed and other supplies to farmers. Heather purchases a $20,000 tractor from Kenny and pays Kenny with $18,000 in cash and $2,000 in corn. How much gross income...
-
Name five techniques you can use to ensure that visual aids do not distort graphic information.
-
The normal strain in the 45° direction on the surface of a circular tube (see figure) is 880 ( 10-6 when the torque T = 750 lb-in. The tube is made of copper alloy with G = 6.2 ( 106 psi. If the...
-
(a) What initial promotional plan directed to consumers in the target market did Callaway use? (b) Why did this make sense to Callaway and her team when Warm Delights was launched?
-
The Spring family has owned and operated a garden tool and implements manufacturing company since 1952. The company sells garden tools to distributors and also directly to hardware stores and home...
-
Selected ledger accounts for Realm Company are given below for the just completed year: Raw Materials Credits Bal. 1/1 Debits 41,000 475,000 Bal.31/12 82,000 Manufacturing Overhead Debits 427,400...
-
Consider the reaction analyzed in Study Problem 3.2 (p. 91), reproduced below. Identify the nucleophilic center, the electrophilic center, and the leaving group in the forward direction. (Dont...
-
Offer an explanation for each of the following observations. (a) Compound A exists mostly in a chair conformation with an equatorial OH group, but compound B prefers a chair conformation with an...
-
What other benefits will accrue with smart grids?
-
I need help with discussion posts that respond to 3 of these comments. 2 of them being the first on each picture. RUBRIC: articles to mention Coleman, R., & Banning, S. (2006). Network TV news'...
-
2. Best Use of Scarce Resource DigiCom Corporation produces three sizes of television sets: 12-inch screen, 26-inch screen, and 40-inch screen. Revenue and cost information per unit for each product...
-
Gunther invested $15,000 into a segregated fund with a 65% maturity guarantee 10 years ago. The fund is now maturing and has a current market value of $22,261. Gunther decides to withdraw his...
-
(a) Consider the following financial data (in millions of dollars) for Costello Laboratories over the period of 2014-2018: Year Sales Net income Total assets Common equity 2014 $3,800 $500 $3,900...
-
The Pizza Pie 'N Go sells about 2300 one-topping pizzas each month. The circle graph displays the most requested one-topping pizzas, by percentage, for one month. Most Popular One-Topping Pizzas...
-
Define paravertebral ganglion.
-
The senior management at Davis Watercraft would like to determine if it is possible to improve firm profitability by changing their existing product mix. Currently, the product mix is determined by...
-
This alkynes hydration reaction can occur without added Hg2+. Show all the steps in themechanism. H,SO, PhC=CH + H20 PHCCH3
-
Explain which compound has a faster rate of reaction withHCI: b) or or NO2 or
-
The addition of C1 to (E)-2-pentene produces a recemic mixture of (2R,3S)-2,3- dichloropentane and its enantiomers, 2R,3S)-2,3- dichloropentane. (a) Show the structure of the two chloronium ions that...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


