Acetals can be used as protecting groups for alcohols. One such protecting group is the tetrahydropyranyl ether
Question:
Acetals can be used as protecting groups for alcohols. One such protecting group is the tetrahydropyranyl ether (THP ether).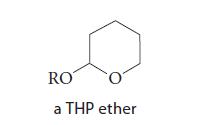
THP ethers are introduced by treating an alcohol with dihydropyran and p-toluenesulfonic acid catalyst.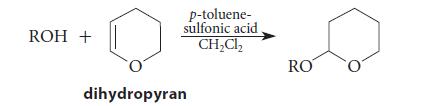
THP ethers are stable to base but are rapidly removed by dilute aqueous acid.
(a) Give the structure of the product formed (in addition to the alcohol ROH) when a THP ether is treated with aqueous acid.
(b) Using the THP protecting group as part of your strategy, outline a synthesis of 2-methyl-2,6- hexanediol from 4-bromo-1-butanol.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





