Alkenes can undergo the addition of hydrogen in the presence of certain catalysts. The H of this
Question:
Alkenes can undergo the addition of hydrogen in the presence of certain catalysts.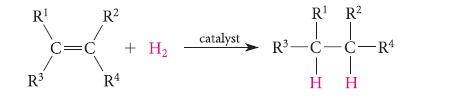
The ΔH° of this reaction, called the enthalpy of hydrogenation, can be measured very accurately and can serve as a source of heats of formation. Consider the following enthalpies of hydrogenation: (E)-3-hexene, 2117.9 kJ mol–1 (28.2 kcal mol–1); (Z)-3-hexene, 2121.6 kJ mol–1 (29.1 kcal mol–1). Calculate the heats of formation of these two alkenes, given that the ΔH°f of hexane is 2167.2 kJ mol–1 (40.0 kcal mol–1).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





