Although enols are unstable compounds (Sec. 14.5A), suppose that the acidity of an enol could be measured.
Question:
Although enols are unstable compounds (Sec. 14.5A), suppose that the acidity of an enol could be measured. Which would be more acidic: enol A or alcohol B? Why?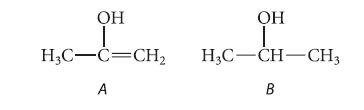
Transcribed Image Text:
OH OH I H3C-C=CH₂ H3C-CH-CH3 A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The enol A is more acidic by about five pK a units ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
There are four diastereomers (A D, margin) of (4S)-2-bromo-4-phenylcyclohexanol. As a team, formulate their structures and draw each diastereomer in the most stable chair conformation (see Table...
-
The following Excel output summarizes the results of an analysis of variance experiment in which the treatments were three different hybrid cars and the variable measured was the miles per gallon...
-
Use the chart of accounts you created in Chapter 1 (and add accounts where necessary) All of the first months activity for Shine King Cleaning is as follows. Nov 1 Evan Hudson deposited $35,000 in...
-
During the "Self-assessment continuum", what are two major red flags?
-
Many emerging countries have political instability, what are the consequences of this? LO.1
-
Inez has a specific set of plans to build a sailboat. The plans are detailed, and any boatbuilder can construct the boat. Inez secures bids, and the low bid is made by the Whale of a Boat Corp. Inez...
-
The adjusted trial balance for Tybalt Construction on December 31 of the current year follows. Credit $ 22,500 60,000 YRAT CONSTRUCTION Adjustad Teal Balance December 31 Account Titis Debit 101 Cash...
-
Enols, like phenols, have pK a values of ~1011. However, the pK a of warfarin, a widely used anticoagulant, is 5.0. (a) Give the structure of the conjugate base of warfarin. (b) Use resonance...
-
Give the products formed in the reaction of 1-hexene with each of the following compounds in the presence of the Grubbs G2 catalyst. (a) An excess of allyl alcohol (2-propen-1-ol) (b) An excess of...
-
The following income statement applies to Stuart Company for the current year: .:. Required a. Use the contribution margin approach to calculate the magnitude of operating leverage. b. Use the...
-
On Apple company with specific iPhone product Required to conduct a SWOT and PESTEL analysis, identifying the internal strengths and weaknesses and external opportunities and threats of the Apple...
-
In which social platforms are Walmart's brand/company active? In your opinion, are they doing a good job regarding customer engagement through social media channels? (Required: screenshots from the...
-
After you have watched both films, how would you describe each film? Also, consider what makes these early films different. List as many observations as you can that separate the Lumi re brothers...
-
How to develop the following points with the Poshmark application for second hand? 1. What are the main reasons for using this product? Or why not? 2. What are the hidden motivations? 3. Are there...
-
Suppose, in an experiment to determine the amount of sodium hypochlorite in bleach, you titrated a 22.84 mL sample of 0.0100 M K I O 3 with a solution of N a 2 S 2 O 3 of unknown concentration. The...
-
(a) Prove that every eigenvalue of a matrix A is also an eigenvalue of its transpose AT. (b) Do they have the same eigenvectors? Prove that if v is an eigenvector of A with eigenvalue and w is an...
-
Give an example of transitory income. What effect does this income have on the marginal propensity to consume?
-
Propose a mechanism that can explain the occurrence of this reaction: 0 CH2
-
When acetone is treated with anhydrous ammonia in the presence of anhydrous calcium chloride (a common drying agent), crystalline product C is obtained on concentration of the organic liquid phase of...
-
The difference in positive-charge distribution in an amide that accepts a proton on its oxygen or its nitrogen atom can be visualized with electrostatic potential maps. Consider the electrostatic...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


