An initiation step in the free-radical co-polymerization of styrene and 1,3-butadiene is the free-radical addition of a
Question:
An initiation step in the free-radical co-polymerization of styrene and 1,3-butadiene is the free-radical addition of a peroxidederived radical to a double bond of 1,3-butadiene: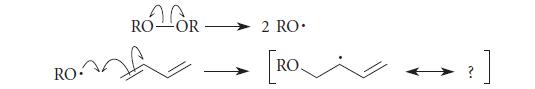
(a) Use the fishhook curved-arrow notation to derive the missing resonance structure.
(b) Use this resonance structure as part of a fishhook mechanism for free-radical co-polymerization to form SBR. (Show the incorporation of one diene unit in a 1,4-addition and one styrene unit.)
(c) Suggest a reason that the diene part of the polymer has mostly trans double-bond stereochemistry.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





