Arrange the labeled bonds in the following molecule in order of increasing length, shortest first. Explain your
Question:
Arrange the labeled bonds in the following molecule in order of increasing length, shortest first. Explain your reasoning.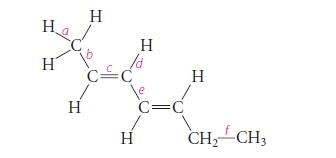
Transcribed Image Text:
Ha H Η Η όξι Η H C=C H CH,CH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The order of increasing bond length is d a 21s bond is slightly shorter ...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following bonds in order of increasing stretching frequencies, and explain your reasoning. C=C C=C C=0 C-C
-
Arrange each of the following in order of increasing acidity. You may need to use a couple of rules to decide the order for a given series. Explain the reasoning you use in each case. a. HBrO2,...
-
Arrange the following compounds in order of increasing boiling point. Explain your answer in terms of the intermolecular forces in each compound. (a) (b) (c) (d)
-
1. As a policy maker you should never worry much about those are eligible for Medicaid benefits and do not enroll. This is because they will enroll in public insurance if they need it. True or False?...
-
Multiple Choice Questions 1. A valid defense in a defamation suit is A. Falseness B. Honest error C. Improbability D. Opinion E. Third-party reliance 2. Joe Student, irate that on an exam he received...
-
What hybridization do you expect for the atom indicated in red in each of the following species? (a) CH3CO2- (b) PH4+ (c) AlF3 (d) H2C==CH-CH2+
-
What is the purpose of the final meeting and presentation? AppendixLO1
-
The MSU football team has 10 games scheduled for next season. The business manager wishes to estimate how much money the team can be expected to have left over after paying the season's expenses,...
-
Assume that an oil company is being sued for an oil spill that occurred at sea. The company denies any wrong-doing, but a class-action suit against the company has been initiated by several parties....
-
Name the following compound using IUPAC substitutive nomenclature. HC=CCH,CH,CH, I CH,CH,CH,CH,CH,
-
Naphthalene can be described by two resonance structures in addition to the following structure. Derive these structures with the curved-arrow notation. two additional structures naphthalene
-
In Exercises write an equation for the specified line. With slope - 3 and y-intercept 3
-
Required: Prepare the supporting schedules for your portfolio for presentation to Mandla the supervisor and senior administrator. The schedules for the portfolio need to cover the following: Part A...
-
Write a program that will predict the size of a population of organisms. The program should ask for the starting number of organisms, their average daily population increase (as a percentage), and...
-
Management is keen to reduce inventory levels for materials as well and closing inventories are to be much lower. Expected levels are shown below: Material M1 Material M2 Material M3 2,200 kg 1,300...
-
How do you calculate incremental cost for the following: Complying with the Clean Air Act Amendments will be costly. There are three main options for complying with the Clean Air Act: analyze the...
-
How do I journalize this transaction? Mountain Swirl Ice Cream purchased and took delivery of one ice cream machine for $7,500. Record the sale and the cost of the sale. Markup is 150% of cost....
-
Exposure to tobacco smoke immobilizes and destroys cilia, how might this effect explain why smokers have an increased incidence of coughing and respiratory infections?
-
Draw two scatterplots, one for which r = 1 and a second for which r = 21.
-
Which is more stable, a 1, 4-trans disubstituted cyclohexane or its cis isomer?
-
Cis-l, 2-Dimnethylcyclohutane is less stable than its transisomer, hut cis-1, 3- dimethylcyclobutanc is more stable than its transisomer. Draw the most stable conformations of both, and explain.
-
Draw the two chair conformations of cis-1-chloro-2-methylcyclohexane. Which is more stable, and by how much?
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


