At 100 C, d-idose exists mostly (about 86%) as a 1,6-anhydropyranose: (a) Draw the chair conformation of
Question:
At 100 °C, d-idose exists mostly (about 86%) as a 1,6-anhydropyranose: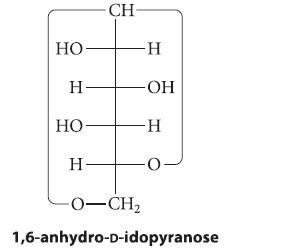
(a) Draw the chair conformation of this compound.
(b) Explain why d-idose has more of the anhydro form than d-glucose. (Under the same conditions, glucose contains only 0.2% of the 1,6-anhydro form.)
Transcribed Image Text:
НО Н- НО Н- CH -Н -ОН -Н -O-CH2 1,6-anhydro-D-idopyranose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a b The anhydro form of Didose has the following chair conformation CH HO 16anhydroDidopyranose OH H...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Problems 1956, solve each system of equations. If the system has no solution, state that it is inconsistent. 2x - 3y z = 0 3x + 2y + 2z = 2 x + 5y + 3z = 2
-
In Problems 43 52, graph each system of linear inequalities. State whether the graph is bounded or unbounded, and label the corner points. x 0 y 0 3x + y 6 2x + y 2
-
The number of admissions to all types of hospitals in a country between 1989 and 1996 can be described by the function A(t) = 35.283t2-1053.78t+40,539.967 thousand people where t is the number of...
-
On January 1, 2014, Iron Mountain Ski Corporation purchased a new snow-grooming machine for $50,000. The machine is estimated to have a 10-year life with a $2,000 salvage value. What journal entry...
-
A sample of a radioactive isotope is found to have an activity of 115.0 Bq immediately after it is pulled from the reactor that formed it. Its activity 2 h 15 min later is measured to be 85.2 Bq. (a)...
-
A concave mirror brings the suns rays to a focus in front of the mirror. Suppose the mirror is submerged in a swimming pool but still pointed up at the sun. Will the suns rays be focused nearer to,...
-
Lottery Number Selection A lottery has 52 numbers. In how many different ways can 6 of the numbers be selected? (Assume that order of selection is not important.)
-
The UFSU Corporation intends to borrow $450,000 to support its short-term financing requirements during the next year. The company is evaluating its financing options at the bank where it maintains...
-
Milo Clothing experienced the following events during Year 1, its first year of operation: 1. Acquired $18,500 cash from the issue of common stock. 2. Purchased inventory for $5,000 cash. 3. Sold...
-
The proton NMR of the C1 proton region of D-glucopyranose shows that both anomers are present. (The large peak in the middle is residual HDO in the H 2 O solvent.) The integrals are given in...
-
L-Ascorbic acid (vitamin C) has the following structure: (a) Ascorbic acid has pK a = 4.21, and is thus about as acidic as a typical carboxylic acid. Identify the acidic hydrogen and explain. (b)...
-
Marco Brolo is one of three partners who own and operate Silkroad Partners, a global import-export business. Marco is the partner in charge of recording partnership transactions in the accounts. On...
-
1. How can the use of MRP contribute to profitability? 2. What are some of the unforeseen costs of ERP implementation? Discuss.
-
The COVID-19 pandemic resulted in strains on the healthcare supply chain. Discuss why supply chain management (SCM) is important to healthcare organizations. Furthermore, explain how healthcare...
-
Financial reporting quality relates to the quality of the information regarding the financial wellbeing of a firm that is contained in financial reports, including note disclosures. The quality of...
-
Exercises: Situational questions. Each answer must be supported by a legal basis. 1. What is the difference when an employee was dismissed for just cause than he was dismissed for an authorized...
-
In international trade, what is the difference between a contracted agent in a foreign country and a contracted distributor?What are some of the key issues that can arise in the relationship between...
-
An electron moving in the positive x -direction passes through a slit of width y = 85 nm. What is the minimum uncertainty in the electron's velocity in the y -direction?
-
Determine the center and radius of each circle. Sketch each circle. 4x 2 + 4y 2 9 = 16y
-
Propose a structure for the compound A (C6H15O2 N) that is unstable in aqueous acid and has the following NMR spectra: 2.30 (6H, s): 2.45 (2H. d,J Hz); 3.27 (6H, s): 4.50 (1 H. t. 6 Hz) 6 Proton...
-
Give a curved-arrow mechanism for each of the reamangementreactions.
-
A chemist, Mada Meens, treated ammonia with pentanal in the presence of hydrogen gas and a catalyst in the expectation of obtaining l-pentanamine by reductive amination, In addition to l-pentanamine,...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


