Complete the reactions given in Fig. P19.48 by giving the principal organic product(s). @ (b) (d) O
Question:
Complete the reactions given in Fig. P19.48 by giving the principal organic product(s).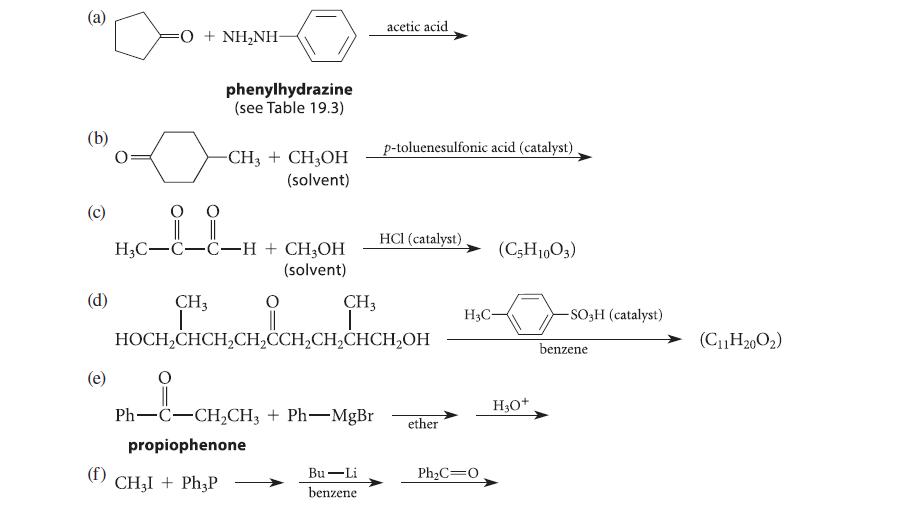
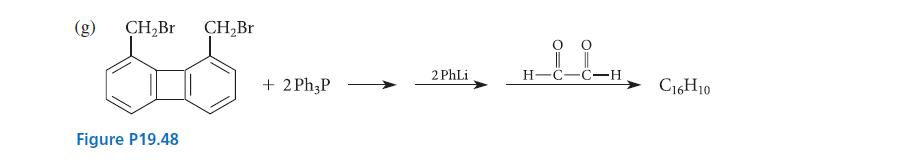
Transcribed Image Text:
@ (b) (d) O + NHẠNH CH3 I phenylhydrazine (see Table 19.3) 0 0 H3C-C-C-H + CH3OH (solvent) -CH3 + CH3OH (solvent) CH3I + Ph3P CH3 T O || Ph–C–CH,CH3 + Ph—MgBr propiophenone acetic acid HOCH₂CHCH₂CH₂CCH₂CH₂CHCH₂OH Bu-Li benzene p-toluenesulfonic acid (catalyst) HCI (catalyst) ether H3C- Ph₂C=0 (C5H1003) H₂O+ -SO-H (catalyst) benzene (C₁1H20O₂)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a The reaction is formation of a hydrazone a type of imine see Table 193 NNH b The reaction is a str...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete the reactions given in Fig. P19.45 by giving the principal organic product(s). Fig. P19.45 (a) (b) (c) p-toluenesulfonic acid (catalyst CH, t CH,OH - (solvent) ether Hio -caphenone, +...
-
Complete the reactions given in Fig. P22.81 by giving the major organic products. Explain your reasoning. NaOEt excess) EtOH H,o heat CHsI (c) CI NO, H ot, heat C CH LiAIH 2) HaO (e) CH Et CHCHCOEOH...
-
Complete the reactions given in Fig. P21.52 by giving the principal organic products. Explain how you arrived at your answers. NaOH CH O (trace) H,C CCHO CH +CH OH (solvent) Ph NH2 1 (CgH,NO3)...
-
Finally, Reza mentions that he was hired into the CFO role on an interim basis with the possibility of being made permanent based on performance. Although he wants to succeed in this role, he does...
-
It is December 31 and time for you to close the books for Brett Tilman Enterprises. Requirement 1. Journalize the closing entries for Brett Tilman Enterprises: a. Service revenue, $20,600. b. Make a...
-
Businesses and consumers in China process more than $1 trillion (U.S. equivalent) in electronic payments every year. The United States claimed that the Chinese government used several regulatory...
-
Japanese IQ scores. The Wechsler Intelligence Scale for Children is used (in several languages) in the United States and Europe. Scores in each Chapter 13 Exercises 285 case are approximately...
-
Cost-Cutting Proposals Chatman Machine Shop is considering a four-year project to improve its production efficiency. Buying a new machine press for $530,000 is estimated to result in $205,000 in...
-
Estate recovery program are design to
-
Give the structures of the two separable isomers formed in the following reaction. OH OH +PhCH=0 acid catalyst
-
Each of the reactions shown in Fig. P19.44 gives a mixture of two separable isomers. What are the two isomers formed in each case? (a) HC. + LiAlH4 (b) PhCH O + HC-CH=PPh3 Figure P19.44 H3O+
-
The information below pertains to the retiree health care plan of Thompson Technologies: Thompson began funding the plan in 2018 with a contribution of $127,000 to the benefit fund at the end of the...
-
Given the following differential equation, dydx = sin ( x + y ) Find the following: ( a ) The substitution u = ( b ) The transformed differential equation dudx = ( c ) The implicit solution, given...
-
Consider the following type declarations TYPE Alinteger; A2 pointer to float; A3 pointer to integer; T1 structure (x: integer; } T2 structure (x: A1; next pointer to integer; } b float; } a :...
-
https://www.viddler.com/embed/82b62f65 Questions: How do companies decide where to locate their facilities? Why has just-in-time inventory control become a dominant production process used in the...
-
Adjusting Entries for Interest At December 31 of Year 1, Portland Corporation had two notes payable outstanding (notes 1 and 2). At December 31 of Year 2, Portland also had two notes payable...
-
We want to get an idea of the actual mass of 235U involved in powering a nuclear power plant. Assume that a single fission event releases 200 MeV of thermal energy. A 1,000 MWe electric power plant...
-
The Spectral Decomposition: (i) Let A be a symmetric matrix with eigenvalues λ1,... ,λn and corresponding orthonormal eigenvectors μ1, ...,μ1....
-
Design a circuit which negative the content of any register and store it in the same register.
-
Compound A (C9H18O) forms a phenylhydrazone, but it gives a negative Tollens' test. The IR spectrum of A has a strong band near 1710 cm-1. The broadband proton decoupled 13C NMR spectrum of A is...
-
Compound B (C8H12O2) shows a strong carbonyl absorption in its IR spectrum. The broadband proton-decoupled 13C NMR spectrum of B has only three signals, at 19 (CH3), 71 (C), and 216 (C). Propose a...
-
(a) What would be the frequencies of the two absorption bands expected to be most prominent in the infrared spectrum of 4-hydroxycycloheptanone (C)? (b) In reality, the lower frequency band of these...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


