Complete the reactions given in Fig. P23.65 by giving the structure(s) of the major product(s). Explain how
Question:
Complete the reactions given in Fig. P23.65 by giving the structure(s) of the major product(s). Explain how you arrived at your answers.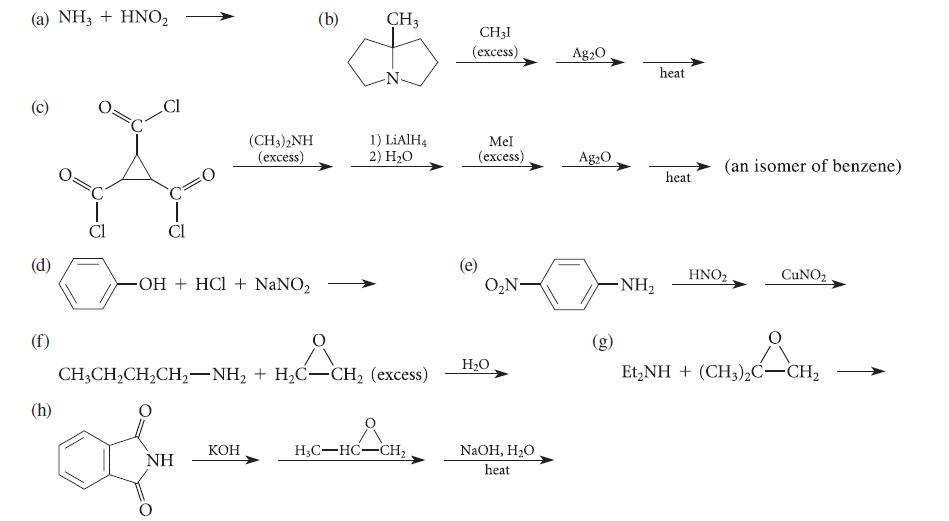
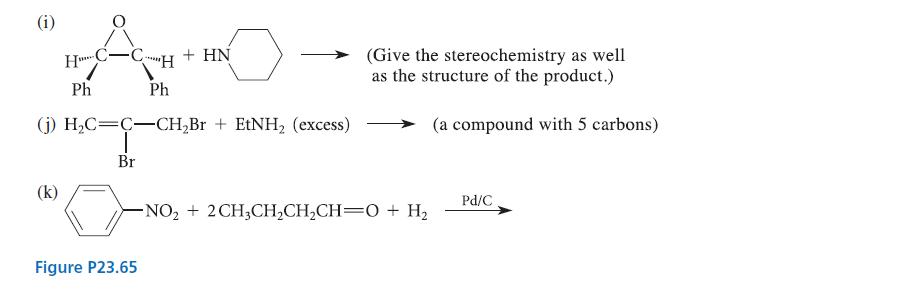
Transcribed Image Text:
(a) NH3 + HNO₂ (d) (f) (h) Cl CI Cl -OH + HCl + NaNO₂ (CH3)2NH (excess) NH KOH CH3 Œ H&CH CH3CH₂CH₂CH₂-NH₂ + H₂C-CH₂ (excess) 1) LiAlH4 2) H₂O H3C HC-CH₂ CH3I (excess) Mel (excess) O₂N- H₂O NaOH, H₂O heat Ag₂O Ag₂O -NH₂ heat heat (an isomer of benzene) HNO₂ CuNO₂ Et,NH + (CH3) C—CH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a b Since diazotization of RNH gives N and products derived from R then diazotization of HNH should give H that is HO and N Exhaustive methylation is ...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete the reactions given in Fig. P21.52 by giving the principal organic products. Explain how you arrived at your answers. NaOH CH O (trace) H,C CCHO CH +CH OH (solvent) Ph NH2 1 (CgH,NO3)...
-
Complete the reactions given in Fig. P22.81 by giving the major organic products. Explain your reasoning. NaOEt excess) EtOH H,o heat CHsI (c) CI NO, H ot, heat C CH LiAIH 2) HaO (e) CH Et CHCHCOEOH...
-
Complete the reactions given in Fig. P19.45 by giving the principal organic product(s). Fig. P19.45 (a) (b) (c) p-toluenesulfonic acid (catalyst CH, t CH,OH - (solvent) ether Hio -caphenone, +...
-
If a currency reform has no effects on the economys real variables, why do governments typically institute currency reforms in connection with broader programs aimed at halting runaway inflation?...
-
Betsy Ray started an accounting service on June 1, 20--, by investing $20,000. Her net income for the month was $10,000, and she withdrew $8,000. Prepare a statement of owners equity for the month of...
-
Identify and briefly describe the basic business processes included within the scope of product life cycle management (PLM).
-
State the possible reasons for difference in profits shown by financial accounts and cost accounts.
-
Granite Stone Creamery sold ice cream equipment for $ 16,000. Granite Stone originally purchased the equipment for $ 90,000, and depreciation through the date of sale totaled $ 71,000. What was the...
-
Record the journal entry for these transactions. Note: If no entry is required for a transaction/event, select "No joumal entry required" in the first account field. Journal entry worksheet The...
-
Outline a synthesis for each of the following compounds from the indicated starting materials and any other reagents. The starting material for the compounds in parts (a) through (e) is pentanoic...
-
Imagine that you have been given a sample of racemic 2-phenylbutanoic acid. Outline steps that would allow you to obtain pure samples of each of the following compounds from this starting material...
-
8. Use the retail method of inventory to find the cost of goods sold and the cost of ending inventory using the table in Exercise 1, the following table, and the fact that sales are $987. Date of...
-
Factor the expression. 4x+31x+21
-
What was the total cost of Job #1253 for January? * (1 Point) BREAD Co. is a print shop that produces jobs to customer specifications. During January 2019, Job #1253 was worked on and the following...
-
The Greensboro Performing Arts Center (GPAC) has a total capacity of 7,600 seats: 2,000 center seats, 2,500 side seats, and 3,100 balcony seats. The budgeted and actual tickets sold for a Broadway...
-
eBook Current position analysis The bond indenture for the 10-year, 9% debenture bonds issued January 2, 2015, required working capital of $100,000, a current ratio of 1.5, and a quick ratio of 1 the...
-
Explain Below terms 1-Leverage Ratios 2-Profitability Ratios 3-Market Value Ratios 4-Liquidity Ratios 5-Efficiency Ratios
-
A bar of length = 2 has a constant stiffness function c = 1/3. Find and graph the displacement and stress when the bar is subjected to a force f(x) = 1 - x and both ends are fixed at their...
-
A crop-dusting plane flies over a level field at a height of 25 ft. If the dust leaves the plane through a 30 angle and hits the ground after the plane travels 75 ft, how wide a strip is dusted? See...
-
(a) Is trans-1, 2-dimethylcyclopentane (5) superposable on its mirror image (i.e., on compound 6)? (b) Is cis-1, 2-dimethylcyclopentane (7) superposable on its mirror image? (c) Is cis-1,...
-
Write structural formulas for all of the stereoisomers of 1, 3-dimethylcyclopentane. Label pairs of enantiomers and meso compounds if they exist.
-
Write formulas for all of the isomers of each of the following. Designate pairs of enantiomers and achiral compounds where they exist. (a) 1-Bromo-2-chlorocyclohexane (b) 1-Bromo-3-chlorocyclohexane...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


