Compound A, C 11 H 12 O, which gave a negative Tollens test, was treated with LiAlH
Question:
Compound A, C11H12O, which gave a negative Tollens test, was treated with LiAlH4, followed by dilute acid, to give compound B, which could be resolved into enantiomers.
When optically active B was treated with CrO3 in pyridine, an optically inactive sample of A was obtained.
Heating A with hydrazine in base gave hydrocarbon C, which, when heated with alkaline KMnO4, gave carboxylic acid D. Identify all of the compounds and explain your reasoning.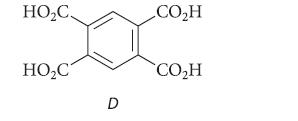
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





