Design a synthesis of methyl orange (Eq. 23.49) using aniline as the only aromatic starting material. -N=N-
Question:
Design a synthesis of methyl orange (Eq. 23.49) using aniline as the only aromatic starting material.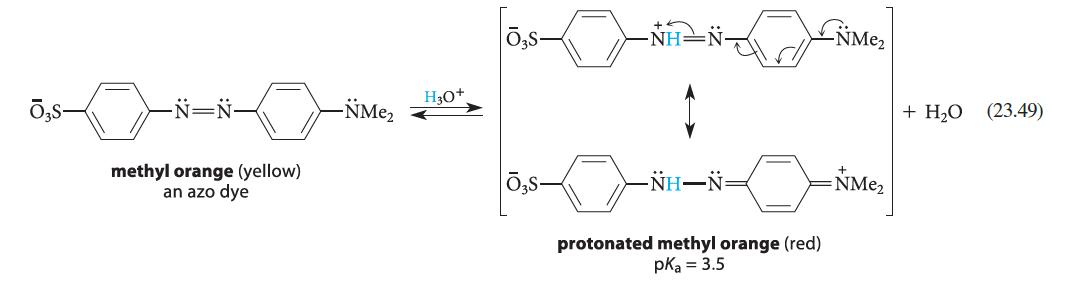
Transcribed Image Text:
-N=N- methyl orange (yellow) an azo dye -NMe₂ H3O+ NH–N protonated methyl orange (red) pka = 3.5 NME₂ =NMe₂ + H₂O (23.49)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
pAminobenzenesulfonic acid is prepared and diazotized to give the diazonium ion A This syn...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the flow rate in each tube of the system shown. The pipes are cast iron; the flow rate in the shunts is expressed in liters/sec. 9.5 159.3 305 m 458m 9.5 11 203mm 458m 305m 254mm 203mm 153m...
-
Using benzene or toluene as the only aromatic organic starting material, devise a synthesis for each of the following compounds. Name the product. CH CH3 Cl CH3 Cl a. b. C. d. Br NO2 NO2
-
Design a synthesis of 3-pentyn-1-ol using propyne and ethylene oxide as the only sources of carbon atoms.
-
Abhishek Ltd. is manufacturing cotton clothes. It has been consistently earning good profits for many years. This year too, it has been able to generate enough profits. There is availability of...
-
Match the following users with the information needed. 1. Owners 2. Managers 3. Creditors 4. Government agencies a. Whether the firm can pay its bills on time b. Detailed, up-to-date information to...
-
Suppose the central bank is following a constant-money-growth-rate rule and the economy is hit with a severe economic downturn. Use an aggregate supply and demand graph to show the possible effects...
-
SV Ltd. has furnished you the following information from the financial books for the year ended 31 March 1998. Profit and loss account for the year ended 31 March 1998 Opening stock Rs Sales Rs 500...
-
Use the data in 401KSUBS.RAW for this question, restricting the sample to fsize = 1. (i) To the model estimated in Table 8.1, add the interaction term, e401k ( inc. Estimate the equation by OLS and...
-
A stock price is currently $50 and it follows geometric Brownian motion with an expected return of 10% and volatility of 25% per year. The probability that the stock price at the end of 9 months will...
-
(a) Which one of the following three amines can be prepared by either the Gabriel synthesis or the Staudinger reaction: 2,2-dimethyl-1-propanamine, 3-methyl-1-pentanamine, or N-butylaniline? (b)...
-
(a) Write a Lewis structure for HNO 2 in Eq. 23.50. (b) Write a mechanism for the reaction shown in Eq. 23.50. Me,NH + HNO, + HO MeN N,N-dimethylnitrosamine (89-90% yield) (23.50)
-
A Question of Ethics Beverly Tull had worked for Atchison Leather Products, Inc., in Kansas for ten years when, in 1999, she began to complain of hand, wrist, and shoulder pain. Atchison recommended...
-
Task 2 In addition to the report produced for Task 1, the SMT have asked that you produce a short presentation, (minimum of 2 slides per bullet point), to help ensure that employees handle, store and...
-
Real solutions for x 2 = 5 ( x + 3 6 0 ) ?
-
1) Two-stage compressor with irreversibilities = You need to build a two-stage compression system with intercooling to increase the pressure of Argon (monatomic gas, constant specific heat) from pi...
-
A tightrope is connected at each end to a vertical tree trunk at a height of 1.57 meter above the ground. The two trees are located a distance 5.00 meters apart. At the midpoint of the tightrope, a...
-
A missing order occurs when a maximum of the two-slit diffraction pattern lines up with the minimum of the single slit diffraction pattern. Adjust the parameters of the simulation to create a...
-
What is the effect of tridiagonalization on the eigenvectors of the matrix?
-
You are standing on the top of a building and throw a ball vertically upward. After 2 seconds, the ball passes you on the way down, and 2 seconds after that, it hits the ground below. a. What is the...
-
Draw a hypothetical free-energy diagram for the SN2 reaction of iodide anion with 1-chlorobutane. Label the diagram as in Fig. 6.4, and assume it is exergonic but without specific values for G¡...
-
When ethyl bromide reacts with potassium cyanide in methanol, the major product is CH3CH2CN. Some CH3CH2NC is formed as well, however. Write Lewis structures for the cyanide ion and for both products...
-
Give structures for the products of each of the following reactions: (a) (b) (c) (d) (e) Nal (1 mol) H -acetone GH,H + NaBr Nal (1 mol), CH,NaCl CI CI BrBr (1 mol C4HS2 2 NaBr OH NaH (-H2) Cl Et O...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


