Estimate each of the bond angles and order the bond lengths (smallest first) in the following molecule.
Question:
Estimate each of the bond angles and order the bond lengths (smallest first) in the following molecule. State any points of ambiguity and explain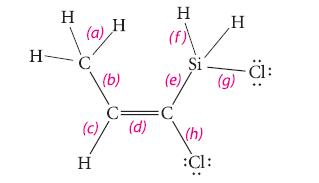
Transcribed Image Text:
H- H (a) C H (b) C= (c) (d) H H (e), =C Si \(h) :Cl: H (g) -ä:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Bond angles bc bd cd de dh and eh are all about 120 bec...View the full answer

Answered By

Dinesh F
I have over 3 years of professional experience as an assignment tutor, and 1 year as a tutor trainee.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Estimate each of the bond angles and order the bond lengths (smallest first) for each of the following molecule. (a) a) (b) d) Br:
-
The following are frequently performed audit procedures for the verification of bonds payable issued in previous years: 1. Analyze the general ledger account for bonds payable, interest expense, and...
-
As Marcia learns more about authentic leadership, she discovers that one of the characteristics that defines authentic leaders is the ability to disclose and share information about themselves...
-
FIGURE P15.62 is a top view of an object of mass m connected between two stretched rubber bands of length L. The object rests on a frictionless surface. At equilibrium, the tension in each rubber...
-
Why is a decision matrix a valuable managerial tool?
-
A framework ABC consists of two rigid bars AB and BC, each having length b (see the first part of the figure). The bars have pin connections at A, B, and C and are joined by a spring of stiffness k....
-
Explain how marketing managers allocate resources.
-
A local partnership is liquidating and is currently reporting the following capital balances: Angela, capital (50% share of all profits and losses) . . . . . . . . . . . $ 19,000 Woodrow, capital...
-
The City of Wolfe issues its financial statements for Year 4 (assume that the city uses a calendar year). The city's general fund is composed of two functions: (1) education and (2) parks. The city...
-
The allyl cation can be represented by the following resonance structures. (a) What is the bond order of each carboncarbon bond in the allyl cation? (b) How much positive charge resides on each...
-
Which of the following orbitals is (are) not permitted by the quantum theory of the hydrogen atom? Explain. 2s 6s 5d 2d 3p
-
Premature to rule out an interest rate increase this year Federal Reserve Bank of New York President William Dudley says that in the current state of the economy, it would be worse for the Fed to...
-
2. (10 points) Two suppliers of products are available to supply the needs of four supermarkets. Each supplier can provide 90 units per day. Each supermarket would like to receive 60 units per day....
-
QUESTION 3 (11 marks) Midrand Ltd acquired a 90% interest in Bramely Ltd on 2 December 20.21 for R2 million. The consideration was settled as follows: Cash payment, Issue of 100 000 shares to the...
-
1. Prepare a Proforma Income Statement for ACCO 295 Corp. (30 points) Use the same Excel table provided to do the calculations with the class explanation. 1. Selling and administrative expenses were...
-
Sandy Foot Hospital expanded their cardiovascular unit to include more operating rooms. They negotiated a 20-year loan with monthly payments and a large sum of $250,000 due at the end of the loan....
-
Oscillations and Resonance Name Lab Procedure Answer questions in red. Download and run the HTMLS application \"resonance\". Driving force: 30 N Driving equency: 5 rad. '5 Spring constant: 5 - 'Irn...
-
Trace each step in the pathway from the external acoustic meatus to hearing receptors.
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
Suggest a method that could be used to prepare this amine from an alkyl halide: PhCH2CH2NH2
-
Show the products of thesereactions: CH Br NaBH4 LIAIH4 ether b) a) CH,OH CH3
-
Show the products of thesereactions: CH,CI 1) NANH2, NH3 (/), NACN b) CH,C3C- a) 2) CH,CH,CH,Br DMSO Br NaCN c) DMSO 1) NANH, 2) CH,I I) NANH2, NH, (1) d) HC=CH 2) CH,CH,Br DMF Br + I NACN e) Cl- Br...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


