Estimate the standard enthalpies of the reactions shown in Eqs. 5.67 and 5.68. (CH3)3C-O + H-Br tert-butoxy
Question:
Estimate the standard enthalpies of the reactions shown in Eqs. 5.67 and 5.68.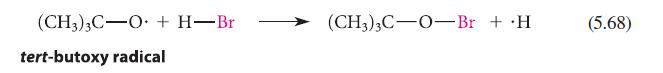
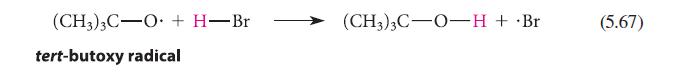
Transcribed Image Text:
(CH3)3C-O + H-Br tert-butoxy radical (CH3)3C-O-Br + .H (5.68)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To obtain the required estimates apply Eq 569 In both equations the bond broken is the HBr bond From Table 53 the bond dissociation energy of this bon...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpies of formation of S(g), F(g), SF4(g), and SF6(g) are 1278.8 kJ/ mol, 179.0 kJ/ mol, 775 kJ/ mol, and 1209 kJ/ mol, respectively. a. Use these data to estimate the energy of an...
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Refer to the table for Moola at the bottom of this page to answer the following questions. What is the equilibrium interest rate in Moola? What is the level of investment at the equilibrium interest...
-
Harrison Ford Company has been approached by a new customer with an offer to purchase 10,000 units of its model IJ4 at a price of $4 each. The new customer is geographically separated from the...
-
School board officials are debating whether to require all high school seniors to take a proficiency exam before graduating. A student passing all three parts (mathematics, language skills, and...
-
Comparing the Costs of Credit Cards. Bobby is trying to decide between two credit cards. One has no annual fee and an 18 percent interest rate, and the other has a $40 annual fee and an 8.9 percent...
-
Neighborhood Supermarkets is preparing to go public, and you are asked to assist the firm by preparing its statement of cash flows for 2014. Neighborhood's balance sheets at December 31, 2013 and...
-
a. Prepare Income Statement of Apple Division, with Segment Margin for the last quarter of 2021. b. Compute the return on investment (ROI) of Orange Division for the last quarter of 2021. Evaluate...
-
Give the products expected when each of the following alkenes is subjected to oxymercurationreduction. (a) Cyclohexene (b) 2-methyl-2-pentene (c) Trans-4-methyl-2-pentene
-
Alkenes undergo the addition of thiols at high temperature in the presence of peroxides or other free-radical initiators. The following reaction is an example. Propose a mechanism for this reaction....
-
Padilha Rental Company's general ledger Cash account showed the following transactions during October 2020: Padilha Rental Company informed its bank that the bank charged a service charge twice. The...
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
Midwest Industrial Products Corporation makes two products, Product H and Product L. Product H is expected to sell 50,000 units next year and Product L is expected to sell 10,000 units. A unit of...
-
You continue to work in the corporate office for a nationwide convenience store franchise that operates nearly 10,000 stores. The per- store daily customer count (i.e., the mean number of customers...
-
For each pair of compounds, explain which is the stronger acid?
-
Explain why the compound on the left is a stronger acid than the compound on the right.
-
Provide IUPAC name for these alkenes: CH3 a) CHCHCHCHCH CH3 c) CHCHCHCHCH3 e) CHCH3 CH3 b) CHCHCHCHCH CHCH3 d) CHCHCHCHCCHCHCHCH T CH, CHCH CH, f)
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


