Explain why the following two alcohols each react with HCl to give the same alkyl chloride. EtS
Question:
Explain why the following two alcohols each react with HCl to give the same alkyl chloride.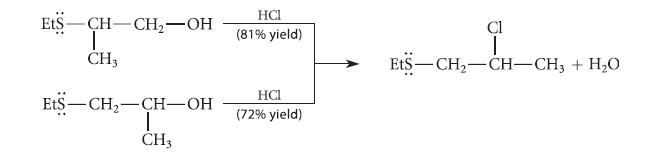
Transcribed Image Text:
EtS -CH-CH₂-OH | CH3 EtS-CH₂-CH-OH T CH3 HCI (81% yield) HC1 (72% yield) Cl ₁+1 EtS—CH,—CH–CH3 + H,O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The two reactions proceed through a common episulfonium ion intermediate that results fro...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give an example of (a) A weak acid that contains oxygen atoms. (b) A weak acid that does not contain oxygen atoms. (c) A neutral molecule that acts as a Lewis acid. (d) A neutral molecule that acts...
-
The equation for the initial reaction between an alcohol (primary or secondary) and iodine solution in the presence of aqueous sodium hydroxide is: RCH(OH)R'() + I2(aq) + 2NaOH(aq) RCOR'(aq) +...
-
Why is trityl chloride much more reactive than the other alkyl halides in Table 17.2? TABLE 17 2 comparison of S,1 Solvolysis Rates of Benzylic and Nonbenzylic Alkyl Halides 25C R-CIHo l + H2O -OH +...
-
In an organization that has high employee satisfaction, ______. Multiple choice question. customer interactions are forced and scarce employee turnover is high more positive interactions take place...
-
Orange Peel, a U.S. company, sold 140,000 cases of tropical fruit to Hanoi Foods, a Vietnamese firm, for 4.25 billion Vietnamese dong. The sale was made on November 17, 2012, when one U.S. dollar...
-
Jill Hansen owns Interior Designs, a furniture store. One of her most popular items is a leather recliner. Following is the recliner inventory activity for August. The recliners on hand at August 1...
-
Explain why managers use marketing dashboards and marketing metrics.
-
Samson Wholesale Beverage Company regularly factors its accounts receivable with the Milpitas Finance Company. On April 30, 2011, the company transferred $800,000 of accounts receivable to Milpitas....
-
Why do manager put such a great amount of emphasis on controlling fixed cost in their organizations?
-
Give the product and its stereochemistry when each of the following alcohols is subjected to asymmetric epoxidation with tert-butyl hydroperoxide, Ti(OiPr) 4 , and the stereoisomer of diethyl...
-
The nucleophilic substitution reaction of sodium 2-bromopropanoate with water shown in Problem 11.35 occurs with retention of configuration at very low NaOH concentrations, but occurs with inversion...
-
In Exercises 1 through 14, compute the indicated values of the given function. h(t) = (2t + 1) 3 ; h(1), h(0), h(1)
-
Solve X+1U6x-13x+2-4x+5
-
Summarize the selected poster's design format, such as the color, layout, font style, size, space, and the subject's analysis format. Also, analyze how the study started. Such as background and...
-
Income statement Prior year Current year Revenues 782.6 900.0 Cost of sales Selling costs Depreciation (27.0) (31.3) Operating profit 90.4 85.7 Interest Earnings before taxes 85.4 78.2 Taxes (31.1)...
-
View the video at the slide title "Lab: Social Media Post" at time 28:20. Link:...
-
Write a program ranges.py in three parts. (Test after each added part.) This problem is not a graphics program. It is just a regular text program to illustrate your understanding of ranges and loops....
-
Graph the function f (x) = cos x + 1/50 sin 50x using the windows given by the following ranges of x and y. (a) -5 x 5, - 1 y 1 (b) - 1 x 1, 0.5 y 1.5 (c) - 0.1 x 0.1, 0.9 y 1.1
-
What is the purpose of the journal wizard?
-
What is the structure of a nonapeptide that gives the following fragments when cleaved? Trypsin cleavage: Val-Val -Pro-Tyr-Leu -Arg, Ser-IIe-Arg Chymotrypsin cleavage: Leu-Arg,...
-
Oxytocin, a nonapeptide hormone secreted by the pituitary gland, functions by stimulating uterine contraction and lactation during childbirth. Its sequence was determined from the following evidence:...
-
Aspartame, a nonnutritive sweetener marketed under the trade name NutraSweet (among others), is the methyl ester of a simple dipeptide, Asp-Phe-OCH3. (a) Draw the structure of aspartame. (b) The...
-
ABC company makes turbo-encabulators, customized to satisfy each customers order. They split overhead into five pools, each with its own activity driver (direct labor for manufacturing, direct labor...
-
Variable manufacturing overhead becomes part of a unit's cost when variable costing is used.Group of answer choicesTrueFalse
-
Santa Fe Corporation has computed the following unit costs for the year just ended:Direct Material used $23Direct Labor $18Fixed selling and administrative cost $18Variable manufacturing overhead...

Study smarter with the SolutionInn App


